+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | yeast TRiC-plp2-actin complex at S4 closed TRiC state | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | TRiC/CCT / actin / phosducin-like protein / CHAPERONE | |||||||||
| Function / homology |  Function and homology information Function and homology informationcellular response to pheromone / negative regulation of chaperone-mediated protein folding / Association of TriC/CCT with target proteins during biosynthesis / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / chaperone mediated protein folding independent of cofactor / chaperonin-containing T-complex / negative regulation of signal transduction / Neutrophil degranulation / ATP-dependent protein folding chaperone / G-protein beta/gamma-subunit complex binding ...cellular response to pheromone / negative regulation of chaperone-mediated protein folding / Association of TriC/CCT with target proteins during biosynthesis / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / chaperone mediated protein folding independent of cofactor / chaperonin-containing T-complex / negative regulation of signal transduction / Neutrophil degranulation / ATP-dependent protein folding chaperone / G-protein beta/gamma-subunit complex binding / unfolded protein binding / protein folding / actin binding / actin cytoskeleton organization / regulation of cell cycle / positive regulation of transcription by RNA polymerase II / ATP hydrolysis activity / ATP binding / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Han WY | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2023 Journal: Sci Adv / Year: 2023Title: Structural basis of plp2-mediated cytoskeletal protein folding by TRiC/CCT. Authors: Wenyu Han / Mingliang Jin / Caixuan Liu / Qiaoyu Zhao / Shutian Wang / Yifan Wang / Yue Yin / Chao Peng / Yanxing Wang / Yao Cong /  Abstract: The cytoskeletal proteins tubulin and actin are the obligate substrates of TCP-1 ring complex/Chaperonin containing TCP-1 (TRiC/CCT), and their folding involves co-chaperone. Through cryo-electron ...The cytoskeletal proteins tubulin and actin are the obligate substrates of TCP-1 ring complex/Chaperonin containing TCP-1 (TRiC/CCT), and their folding involves co-chaperone. Through cryo-electron microscopy analysis, we present a more complete picture of TRiC-assisted tubulin/actin folding along TRiC adenosine triphosphatase cycle, under the coordination of co-chaperone plp2. In the open S1/S2 states, plp2 and tubulin/actin engaged within opposite TRiC chambers. Notably, we captured an unprecedented TRiC-plp2-tubulin complex in the closed S3 state, engaged with a folded full-length -tubulin and loaded with a guanosine triphosphate, and a plp2 occupying opposite rings. Another closed S4 state revealed an actin in the intermediate folding state and a plp2. Accompanying TRiC ring closure, plp2 translocation could coordinate substrate translocation on the CCT6 hemisphere, facilitating substrate stabilization and folding. Our findings reveal the folding mechanism of the major cytoskeletal proteins tubulin/actin under the coordination of the biogenesis machinery TRiC and plp2 and extend our understanding of the links between cytoskeletal proteostasis and related human diseases. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33920.map.gz emd_33920.map.gz | 6.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33920-v30.xml emd-33920-v30.xml emd-33920.xml emd-33920.xml | 29.8 KB 29.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_33920.png emd_33920.png | 76.3 KB | ||
| Filedesc metadata |  emd-33920.cif.gz emd-33920.cif.gz | 8.9 KB | ||
| Others |  emd_33920_half_map_1.map.gz emd_33920_half_map_1.map.gz emd_33920_half_map_2.map.gz emd_33920_half_map_2.map.gz | 49.7 MB 49.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33920 http://ftp.pdbj.org/pub/emdb/structures/EMD-33920 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33920 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33920 | HTTPS FTP |
-Validation report
| Summary document |  emd_33920_validation.pdf.gz emd_33920_validation.pdf.gz | 898.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_33920_full_validation.pdf.gz emd_33920_full_validation.pdf.gz | 898.2 KB | Display | |
| Data in XML |  emd_33920_validation.xml.gz emd_33920_validation.xml.gz | 12.2 KB | Display | |
| Data in CIF |  emd_33920_validation.cif.gz emd_33920_validation.cif.gz | 14.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33920 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33920 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33920 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33920 | HTTPS FTP |
-Related structure data
| Related structure data |  7ylxMC  7yluC  7ylvC  7ylwC  7ylyC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_33920.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33920.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.318 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_33920_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
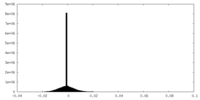
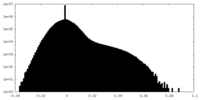
| Density Histograms |
-Half map: #1
| File | emd_33920_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
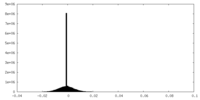
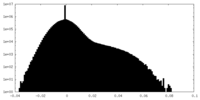
| Density Histograms |
- Sample components
Sample components
+Entire : yeast TRiC-plp2-actin complex at C4 closed TRiC state
+Supramolecule #1: yeast TRiC-plp2-actin complex at C4 closed TRiC state
+Supramolecule #2: TRiC
+Supramolecule #3: plp2
+Macromolecule #1: T-complex protein 1 subunit alpha
+Macromolecule #2: T-complex protein 1 subunit beta
+Macromolecule #3: T-complex protein 1 subunit delta
+Macromolecule #4: T-complex protein 1 subunit epsilon
+Macromolecule #5: T-complex protein 1 subunit gamma
+Macromolecule #6: T-complex protein 1 subunit eta
+Macromolecule #7: T-complex protein 1 subunit theta
+Macromolecule #8: T-complex protein 1 subunit zeta
+Macromolecule #9: Phosducin-like protein 2
+Macromolecule #10: ADENOSINE-5'-DIPHOSPHATE
+Macromolecule #11: MAGNESIUM ION
+Macromolecule #12: ALUMINUM FLUORIDE
+Macromolecule #13: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 38.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: EMDB MAP EMDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.2 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 58195 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller


















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)