[English] 日本語
 Yorodumi
Yorodumi- EMDB-30849: Structure of a human NHE1-CHP1 complex under pH 7.5, bound by car... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-30849 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of a human NHE1-CHP1 complex under pH 7.5, bound by cariporide | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Transporter / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of phosphatase activity / positive regulation of sodium:proton antiporter activity / Sodium/Proton exchangers / regulation of the force of heart contraction by cardiac conduction / cation-transporting ATPase complex / : / Hyaluronan degradation / membrane docking / negative regulation of protein autophosphorylation / regulation of cardiac muscle cell membrane potential ...negative regulation of phosphatase activity / positive regulation of sodium:proton antiporter activity / Sodium/Proton exchangers / regulation of the force of heart contraction by cardiac conduction / cation-transporting ATPase complex / : / Hyaluronan degradation / membrane docking / negative regulation of protein autophosphorylation / regulation of cardiac muscle cell membrane potential / positive regulation of protein transport / cellular response to electrical stimulus / positive regulation of phospholipid biosynthetic process / transporter complex / potassium:proton antiporter activity / positive regulation of action potential / sodium:proton antiporter activity / maintenance of cell polarity / regulation of pH / positive regulation of calcineurin-NFAT signaling cascade / cardiac muscle cell differentiation / sodium ion export across plasma membrane / protein phosphatase 2B binding / membrane organization / microtubule bundle formation / intracellular sodium ion homeostasis / regulation of stress fiber assembly / cardiac muscle cell contraction / sodium ion import across plasma membrane / positive regulation of mitochondrial membrane permeability / response to acidic pH / regulation of cardiac muscle contraction by calcium ion signaling / negative regulation of calcineurin-NFAT signaling cascade / cellular response to acidic pH / regulation of focal adhesion assembly / negative regulation of protein import into nucleus / small GTPase-mediated signal transduction / : / cellular response to cold / positive regulation of cardiac muscle hypertrophy / endoplasmic reticulum-Golgi intermediate compartment / cellular response to antibiotic / positive regulation of the force of heart contraction / negative regulation of protein phosphorylation / protein kinase inhibitor activity / protein complex oligomerization / intercalated disc / positive regulation of protein targeting to membrane / potassium channel regulator activity / negative regulation of protein kinase activity / transport vesicle / response to muscle stretch / phosphatidylinositol-4,5-bisphosphate binding / cytoplasmic microtubule organization / monoatomic ion transport / potassium ion transmembrane transport / cellular response to epinephrine stimulus / T-tubule / proton transmembrane transport / negative regulation of protein ubiquitination / protein export from nucleus / stem cell differentiation / regulation of intracellular pH / potassium ion transport / cellular response to mechanical stimulus / phospholipid binding / kinase binding / cellular response to insulin stimulus / calcium-dependent protein binding / cell migration / lamellipodium / microtubule cytoskeleton / positive regulation of cell growth / cellular response to hypoxia / basolateral plasma membrane / microtubule binding / protein-macromolecule adaptor activity / molecular adaptor activity / membrane fusion / calmodulin binding / apical plasma membrane / protein stabilization / positive regulation of apoptotic process / membrane raft / Golgi membrane / focal adhesion / calcium ion binding / negative regulation of apoptotic process / perinuclear region of cytoplasm / cell surface / endoplasmic reticulum / positive regulation of transcription by RNA polymerase II / mitochondrion / extracellular exosome / nucleoplasm / identical protein binding / membrane / nucleus / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
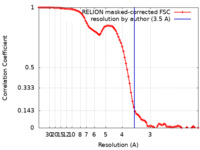
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Dong Y / Gao Y / Li B / Zhang XC / Zhao Y | |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2021 Journal: Nat Commun / Year: 2021Title: Structure and mechanism of the human NHE1-CHP1 complex. Authors: Yanli Dong / Yiwei Gao / Alina Ilie / DuSik Kim / Annie Boucher / Bin Li / Xuejun C Zhang / John Orlowski / Yan Zhao /   Abstract: Sodium/proton exchanger 1 (NHE1) is an electroneutral secondary active transporter present on the plasma membrane of most mammalian cells and plays critical roles in regulating intracellular pH and ...Sodium/proton exchanger 1 (NHE1) is an electroneutral secondary active transporter present on the plasma membrane of most mammalian cells and plays critical roles in regulating intracellular pH and volume homeostasis. Calcineurin B-homologous protein 1 (CHP1) is an obligate binding partner that promotes NHE1 biosynthetic maturation, cell surface expression and pH-sensitivity. Dysfunctions of either protein are associated with neurological disorders. Here, we elucidate structures of the human NHE1-CHP1 complex in both inward- and inhibitor (cariporide)-bound outward-facing conformations. We find that NHE1 assembles as a symmetrical homodimer, with each subunit undergoing an elevator-like conformational change during cation exchange. The cryo-EM map reveals the binding site for the NHE1 inhibitor cariporide, illustrating how inhibitors block transport activity. The CHP1 molecule differentially associates with these two conformational states of each NHE1 monomer, and this association difference probably underlies the regulation of NHE1 pH-sensitivity by CHP1. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_30849.map.gz emd_30849.map.gz | 32.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-30849-v30.xml emd-30849-v30.xml emd-30849.xml emd-30849.xml | 15.8 KB 15.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_30849_fsc.xml emd_30849_fsc.xml | 9.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_30849.png emd_30849.png | 85.4 KB | ||
| Filedesc metadata |  emd-30849.cif.gz emd-30849.cif.gz | 6.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-30849 http://ftp.pdbj.org/pub/emdb/structures/EMD-30849 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30849 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30849 | HTTPS FTP |
-Related structure data
| Related structure data |  7dsxMC  7dsvC  7dswC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_30849.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_30849.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.04 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Human NHE1-CHP1 complex under pH 7.5, bound by cariporide
| Entire | Name: Human NHE1-CHP1 complex under pH 7.5, bound by cariporide |
|---|---|
| Components |
|
-Supramolecule #1: Human NHE1-CHP1 complex under pH 7.5, bound by cariporide
| Supramolecule | Name: Human NHE1-CHP1 complex under pH 7.5, bound by cariporide type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 230 KDa |
-Supramolecule #2: Sodium/protein exchanger 1 (NHE1) under pH 7.5, bound by cariporide
| Supramolecule | Name: Sodium/protein exchanger 1 (NHE1) under pH 7.5, bound by cariporide type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 182 KDa |
-Supramolecule #3: Calcineurin B homologous protein 1 (CHP1) under pH 7.5
| Supramolecule | Name: Calcineurin B homologous protein 1 (CHP1) under pH 7.5 type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|
-Macromolecule #1: Calcineurin B homologous protein 1
| Macromolecule | Name: Calcineurin B homologous protein 1 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 21.36983 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: DEELEEIKKE TGFSHSQITR LYSRFTSLDK GENGTLSRED FQRIPELAIN PLGDRIINAF FPEGEDQVNF RGFMRTLAHF RPIEDNEKS KDVNGPEPLN SRSNKLHFAF RLYDLDKDEK ISRDELLQVL RMMVGVNISD EQLGSIADRT IQEADQDGDS A ASFTEFVK VLEKVDVEQK MSIRFLH UniProtKB: Calcineurin B homologous protein 1 |
-Macromolecule #2: Sodium/hydrogen exchanger 1
| Macromolecule | Name: Sodium/hydrogen exchanger 1 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 57.100363 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: PRKAFPVLGI DYTHVRTPFE ISLWILLACL MKIGFHVIPT ISSIVPESCL LIVVGLLVGG LIKGVGETPP FLQSDVFFLF LLPPIILDA GYFLPLRQFT ENLGTILIFA VVGTLWNAFF LGGLMYAVCL VGGEQINNIG LLDNLLFGSI ISAVDPVAVL A VFEEIHIN ...String: PRKAFPVLGI DYTHVRTPFE ISLWILLACL MKIGFHVIPT ISSIVPESCL LIVVGLLVGG LIKGVGETPP FLQSDVFFLF LLPPIILDA GYFLPLRQFT ENLGTILIFA VVGTLWNAFF LGGLMYAVCL VGGEQINNIG LLDNLLFGSI ISAVDPVAVL A VFEEIHIN ELLHILVFGE SLLNDAVTVV LYHLFEEFAN YEHVGIVDIF LGFLSFFVVA LGGVLVGVVY GVIAAFTSRF TS HIRVIEP LFVFLYSYMA YLSAELFHLS GIMALIASGV VMRPYVEANI SHKSHTTIKY FLKMWSSVSE TLIFIFLGVS TVA GSHHWN WTFVISTLLF CLIARVLGVL GLTWFINKFR IVKLTPKDQF IIAYGGLRGA IAFSLGYLLD KKHFPMCDLF LTAI ITVIF FTVFVQGMTI RPLVDLLAVK KKQETKRSIN EEIHTQFLDH LLTGIEDICG HYGHHHWKDK LNRFNKKYVK KCLIA GERS KEPQLIAFYH KMEMKQAIEL VESGG UniProtKB: Sodium/hydrogen exchanger 1 |
-Macromolecule #3: (1S)-2-{[{[(2R)-2,3-DIHYDROXYPROPYL]OXY}(HYDROXY)PHOSPHORYL]OXY}-...
| Macromolecule | Name: (1S)-2-{[{[(2R)-2,3-DIHYDROXYPROPYL]OXY}(HYDROXY)PHOSPHORYL]OXY}-1-[(PALMITOYLOXY)METHYL]ETHYL STEARATE type: ligand / ID: 3 / Number of copies: 6 / Formula: PGT |
|---|---|
| Molecular weight | Theoretical: 751.023 Da |
| Chemical component information |  ChemComp-PGT: |
-Macromolecule #4: N-[bis(azanyl)methylidene]-3-methylsulfonyl-4-propan-2-yl-benzamide
| Macromolecule | Name: N-[bis(azanyl)methylidene]-3-methylsulfonyl-4-propan-2-yl-benzamide type: ligand / ID: 4 / Number of copies: 2 / Formula: HG0 |
|---|---|
| Molecular weight | Theoretical: 283.347 Da |
| Chemical component information |  ChemComp-HG0: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 6 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK III |
| Details | The NHE1-CHP1 complex was reconstituted into lipid nanodiscs. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Dimensions - Width: 3838 pixel / Digitization - Dimensions - Height: 3710 pixel / Digitization - Frames/image: 1-32 / Number grids imaged: 1 / Number real images: 3855 / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.2 µm / Nominal magnification: 13000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-7dsx: |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)