+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-30310 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human RAD51 post-synaptic complexes mutant (V273P, D274G) | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | meitotic homologous recombination / DNA repair / ATPase / RECOMBINATION | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationpresynaptic intermediate filament cytoskeleton / response to glucoside / mitotic recombination-dependent replication fork processing / DNA recombinase assembly / cellular response to camptothecin / chromosome organization involved in meiotic cell cycle / telomere maintenance via telomere lengthening / double-strand break repair involved in meiotic recombination / nuclear ubiquitin ligase complex / cellular response to cisplatin ...presynaptic intermediate filament cytoskeleton / response to glucoside / mitotic recombination-dependent replication fork processing / DNA recombinase assembly / cellular response to camptothecin / chromosome organization involved in meiotic cell cycle / telomere maintenance via telomere lengthening / double-strand break repair involved in meiotic recombination / nuclear ubiquitin ligase complex / cellular response to cisplatin / DNA strand invasion / mitotic recombination / cellular response to hydroxyurea / DNA strand exchange activity / replication-born double-strand break repair via sister chromatid exchange / lateral element / regulation of DNA damage checkpoint / Impaired BRCA2 binding to PALB2 / telomere maintenance via recombination / single-stranded DNA helicase activity / reciprocal meiotic recombination / Homologous DNA Pairing and Strand Exchange / Defective homologous recombination repair (HRR) due to BRCA1 loss of function / Defective HDR through Homologous Recombination Repair (HRR) due to PALB2 loss of BRCA1 binding function / Defective HDR through Homologous Recombination Repair (HRR) due to PALB2 loss of BRCA2/RAD51/RAD51C binding function / Resolution of D-loop Structures through Synthesis-Dependent Strand Annealing (SDSA) / Resolution of D-loop Structures through Holliday Junction Intermediates / HDR through Single Strand Annealing (SSA) / ATP-dependent DNA damage sensor activity / regulation of double-strand break repair via homologous recombination / nuclear chromosome / Impaired BRCA2 binding to RAD51 / Transcriptional Regulation by E2F6 / replication fork processing / Presynaptic phase of homologous DNA pairing and strand exchange / response to X-ray / ATP-dependent activity, acting on DNA / interstrand cross-link repair / condensed chromosome / DNA polymerase binding / condensed nuclear chromosome / cellular response to ionizing radiation / male germ cell nucleus / meiotic cell cycle / cellular response to gamma radiation / double-strand break repair via homologous recombination / PML body / HDR through Homologous Recombination (HRR) / Meiotic recombination / response to toxic substance / single-stranded DNA binding / site of double-strand break / double-stranded DNA binding / DNA recombination / chromosome, telomeric region / mitochondrial matrix / response to xenobiotic stimulus / DNA repair / DNA damage response / chromatin binding / centrosome / chromatin / nucleolus / perinuclear region of cytoplasm / enzyme binding / ATP hydrolysis activity / protein-containing complex / mitochondrion / nucleoplasm / ATP binding / identical protein binding / nucleus / cytosol / cytoplasm Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
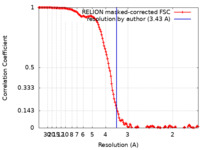
| Method | helical reconstruction / cryo EM / Resolution: 3.43 Å | ||||||||||||
 Authors Authors | Chi HY / Ho MC / Tsai MD / Luo SC / Yeh HY | ||||||||||||
| Funding support |  Taiwan, 3 items Taiwan, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2021 Journal: Nat Commun / Year: 2021Title: Identification of fidelity-governing factors in human recombinases DMC1 and RAD51 from cryo-EM structures. Authors: Shih-Chi Luo / Hsin-Yi Yeh / Wei-Hsuan Lan / Yi-Min Wu / Cheng-Han Yang / Hao-Yen Chang / Guan-Chin Su / Chia-Yi Lee / Wen-Jin Wu / Hung-Wen Li / Meng-Chiao Ho / Peter Chi / Ming-Daw Tsai /  Abstract: Both high-fidelity and mismatch-tolerant recombination, catalyzed by RAD51 and DMC1 recombinases, respectively, are indispensable for genomic integrity. Here, we use cryo-EM, MD simulation and ...Both high-fidelity and mismatch-tolerant recombination, catalyzed by RAD51 and DMC1 recombinases, respectively, are indispensable for genomic integrity. Here, we use cryo-EM, MD simulation and functional analysis to elucidate the structural basis for the mismatch tolerance of DMC1. Structural analysis of DMC1 presynaptic and postsynaptic complexes suggested that the lineage-specific Loop 1 Gln244 (Met243 in RAD51) may help stabilize DNA backbone, whereas Loop 2 Pro274 and Gly275 (Val273/Asp274 in RAD51) may provide an open "triplet gate" for mismatch tolerance. In support, DMC1-Q244M displayed marked increase in DNA dynamics, leading to unobservable DNA map. MD simulation showed highly dispersive mismatched DNA ensemble in RAD51 but well-converged DNA in DMC1 and RAD51-V273P/D274G. Replacing Loop 1 or Loop 2 residues in DMC1 with RAD51 counterparts enhanced DMC1 fidelity, while reciprocal mutations in RAD51 attenuated its fidelity. Our results show that three Loop 1/Loop 2 residues jointly enact contrasting fidelities of DNA recombinases. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_30310.map.gz emd_30310.map.gz | 202.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-30310-v30.xml emd-30310-v30.xml emd-30310.xml emd-30310.xml | 16.8 KB 16.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_30310_fsc.xml emd_30310_fsc.xml | 13.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_30310.png emd_30310.png | 106 KB | ||
| Filedesc metadata |  emd-30310.cif.gz emd-30310.cif.gz | 6.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-30310 http://ftp.pdbj.org/pub/emdb/structures/EMD-30310 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30310 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30310 | HTTPS FTP |
-Related structure data
| Related structure data |  7c9aMC  7c98C  7c99C  7c9cC  7cgyC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_30310.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_30310.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.84 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : RAD51-dsDNA filament (V273P D274G mutant)
| Entire | Name: RAD51-dsDNA filament (V273P D274G mutant) |
|---|---|
| Components |
|
-Supramolecule #1: RAD51-dsDNA filament (V273P D274G mutant)
| Supramolecule | Name: RAD51-dsDNA filament (V273P D274G mutant) / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: DNA repair protein RAD51 homolog 1
| Macromolecule | Name: DNA repair protein RAD51 homolog 1 / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 36.949074 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAMQMQLEAN ADTSVEEESF GPQPISRLEQ CGINANDVKK LEEAGFHTVE AVAYAPKKEL INIKGISEAK ADKILAEAAK LVPMGFTTA TEFHQRRSEI IQITTGSKEL DKLLQGGIET GSITEMFGEF RTGKTQICHT LAVTCQLPID RGGGEGKAMY I DTEGTFRP ...String: MAMQMQLEAN ADTSVEEESF GPQPISRLEQ CGINANDVKK LEEAGFHTVE AVAYAPKKEL INIKGISEAK ADKILAEAAK LVPMGFTTA TEFHQRRSEI IQITTGSKEL DKLLQGGIET GSITEMFGEF RTGKTQICHT LAVTCQLPID RGGGEGKAMY I DTEGTFRP ERLLAVAERY GLSGSDVLDN VAYARAFNTD HQTQLLYQAS AMMVESRYAL LIVDSATALY RTDYSGRGEL SA RQMHLAR FLRMLLRLAD EFGVAVVITN QVVAQPGGAA MFAADPKKPI GGNIIAHAST TRLYLRKGRG ETRICKIYDS PCL PEAEAM FAINADGVGD AKD UniProtKB: DNA repair protein RAD51 homolog 1 |
-Macromolecule #2: DNA (5'-D(P*TP*TP*TP*TP*TP*TP*TP*TP*T)-3')
| Macromolecule | Name: DNA (5'-D(P*TP*TP*TP*TP*TP*TP*TP*TP*T)-3') / type: dna / ID: 2 Details: Authors know the sequence of chains D/E. Chain D: TCA GCT GTT GCC CGT. Chain E: ACG GGC AAC AGC TGA. Since the DNA sequence could not be revealed by the helical reconstruction method, ...Details: Authors know the sequence of chains D/E. Chain D: TCA GCT GTT GCC CGT. Chain E: ACG GGC AAC AGC TGA. Since the DNA sequence could not be revealed by the helical reconstruction method, authors have used poly-T for chain D, poly-A for chain E respectively for model building. Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 2.692778 KDa |
| Sequence | String: (DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT) |
-Macromolecule #3: DNA (5'-D(P*AP*AP*AP*AP*AP*AP*AP*AP*A)-3')
| Macromolecule | Name: DNA (5'-D(P*AP*AP*AP*AP*AP*AP*AP*AP*A)-3') / type: dna / ID: 3 Details: Authors know the sequence of chains D/E. Chain D: TCA GCT GTT GCC CGT. Chain E: ACG GGC AAC AGC TGA. Since the DNA sequence could not be revealed by the helical reconstruction method, ...Details: Authors know the sequence of chains D/E. Chain D: TCA GCT GTT GCC CGT. Chain E: ACG GGC AAC AGC TGA. Since the DNA sequence could not be revealed by the helical reconstruction method, authors have used poly-T for chain D, poly-A for chain E respectively for model building. Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 2.773904 KDa |
| Sequence | String: (DA)(DA)(DA)(DA)(DA)(DA)(DA)(DA)(DA) |
-Macromolecule #4: CALCIUM ION
| Macromolecule | Name: CALCIUM ION / type: ligand / ID: 4 / Number of copies: 3 / Formula: CA |
|---|---|
| Molecular weight | Theoretical: 40.078 Da |
-Macromolecule #5: PHOSPHOAMINOPHOSPHONIC ACID-ADENYLATE ESTER
| Macromolecule | Name: PHOSPHOAMINOPHOSPHONIC ACID-ADENYLATE ESTER / type: ligand / ID: 5 / Number of copies: 3 / Formula: ANP |
|---|---|
| Molecular weight | Theoretical: 506.196 Da |
| Chemical component information |  ChemComp-ANP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 / Component - Concentration: 25.0 mM / Component - Formula: Tris-HCl Details: 25 mM Tris-HCl, pH 7.5, 50 mM KCl and 1 mM dithiothreitol) containing 2 mM AMP-PNP and 5 mM CaCl2 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 200 / Support film - Material: GRAPHENE OXIDE / Support film - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 15 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295 K / Instrument: FEI VITROBOT MARK IV Details: The grids were blotted for 1 sec at 22 degree C with 100% relative humidity and plunge-frozen in liquid ethane cooled by liquid nitrogen using a Vitrobot Mark IV (Thermo Fisher).. |
| Details | protein sample were applied onto a pre-glow-discharged graphene-oxide coated Quantifoil holey carbon grid (1.2/1.3, 200 mesh) using published protocol |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Alignment procedure | Coma free - Residual tilt: 10.0 mrad |
| Specialist optics | Energy filter - Name: GIF Quantum LS / Energy filter - Slit width: 30 eV |
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 3838 pixel / Digitization - Dimensions - Height: 3710 pixel / Digitization - Frames/image: 1-50 / Number grids imaged: 1 / Average exposure time: 5.0 sec. / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal magnification: 165000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)