[English] 日本語
 Yorodumi
Yorodumi- EMDB-29653: Cryo-EM structure of TBAJ-876-bound Mycobacterium smegmatis ATP s... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of TBAJ-876-bound Mycobacterium smegmatis ATP synthase rotational state 1 | |||||||||
 Map data Map data | Cryo-EM structure of TBAJ-876-bound Mycobacterium smegmatis ATP synthase rotational state 1 (unsharpened) | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ATP synthase / mycobacterial / inhibitor / tuberculosis / antibiotic / HYDROLASE / TRANSLOCASE-INHIBITOR complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationproton motive force-driven plasma membrane ATP synthesis / proton-transporting ATP synthase complex, coupling factor F(o) / proton-transporting ATP synthase complex, catalytic core F(1) / H+-transporting two-sector ATPase / proton-transporting ATPase activity, rotational mechanism / proton-transporting ATP synthase activity, rotational mechanism / hydrolase activity / lipid binding / ATP hydrolysis activity / ATP binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria) | |||||||||
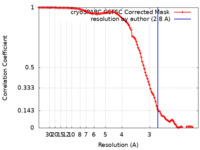
| Method | single particle reconstruction / cryo EM / Resolution: 2.8 Å | |||||||||
 Authors Authors | Courbon GM / Rubinstein JL | |||||||||
| Funding support |  Canada, 1 items Canada, 1 items
| |||||||||
 Citation Citation |  Journal: EMBO J / Year: 2023 Journal: EMBO J / Year: 2023Title: Mechanism of mycobacterial ATP synthase inhibition by squaramides and second generation diarylquinolines. Authors: Gautier M Courbon / Paul R Palme / Lea Mann / Adrian Richter / Peter Imming / John L Rubinstein /   Abstract: Mycobacteria, such as Mycobacterium tuberculosis, depend on the activity of adenosine triphosphate (ATP) synthase for growth. The diarylquinoline bedaquiline (BDQ), a mycobacterial ATP synthase ...Mycobacteria, such as Mycobacterium tuberculosis, depend on the activity of adenosine triphosphate (ATP) synthase for growth. The diarylquinoline bedaquiline (BDQ), a mycobacterial ATP synthase inhibitor, is an important medication for treatment of drug-resistant tuberculosis but suffers from off-target effects and is susceptible to resistance mutations. Consequently, both new and improved mycobacterial ATP synthase inhibitors are needed. We used electron cryomicroscopy and biochemical assays to study the interaction of Mycobacterium smegmatis ATP synthase with the second generation diarylquinoline TBAJ-876 and the squaramide inhibitor SQ31f. The aryl groups of TBAJ-876 improve binding compared with BDQ, while SQ31f, which blocks ATP synthesis ~10 times more potently than ATP hydrolysis, binds a previously unknown site in the enzyme's proton-conducting channel. Remarkably, BDQ, TBAJ-876, and SQ31f all induce similar conformational changes in ATP synthase, suggesting that the resulting conformation is particularly suited for drug binding. Further, high concentrations of the diarylquinolines uncouple the transmembrane proton motive force while for SQ31f they do not, which may explain why high concentrations of diarylquinolines, but not SQ31f, have been reported to kill mycobacteria. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29653.map.gz emd_29653.map.gz | 62.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29653-v30.xml emd-29653-v30.xml emd-29653.xml emd-29653.xml | 27 KB 27 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_29653_fsc.xml emd_29653_fsc.xml | 10.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_29653.png emd_29653.png | 73.4 KB | ||
| Filedesc metadata |  emd-29653.cif.gz emd-29653.cif.gz | 7.2 KB | ||
| Others |  emd_29653_additional_1.map.gz emd_29653_additional_1.map.gz emd_29653_half_map_1.map.gz emd_29653_half_map_1.map.gz emd_29653_half_map_2.map.gz emd_29653_half_map_2.map.gz | 58 MB 116.1 MB 116.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29653 http://ftp.pdbj.org/pub/emdb/structures/EMD-29653 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29653 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29653 | HTTPS FTP |
-Validation report
| Summary document |  emd_29653_validation.pdf.gz emd_29653_validation.pdf.gz | 874.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_29653_full_validation.pdf.gz emd_29653_full_validation.pdf.gz | 874.1 KB | Display | |
| Data in XML |  emd_29653_validation.xml.gz emd_29653_validation.xml.gz | 19 KB | Display | |
| Data in CIF |  emd_29653_validation.cif.gz emd_29653_validation.cif.gz | 24.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29653 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29653 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29653 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29653 | HTTPS FTP |
-Related structure data
| Related structure data |  8g0cMC  8g07C  8g08C  8g09C  8g0aC  8g0bC  8g0dC  8g0eC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_29653.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29653.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of TBAJ-876-bound Mycobacterium smegmatis ATP synthase rotational state 1 (unsharpened) | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.03 Å | ||||||||||||||||||||||||||||||||||||
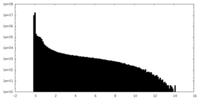
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Cryo-EM structure of TBAJ-876-bound Mycobacterium smegmatis ATP synthase...
| File | emd_29653_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of TBAJ-876-bound Mycobacterium smegmatis ATP synthase rotational state 1 (locally sharpened) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Cryo-EM structure of TBAJ-876-bound Mycobacterium smegmatis ATP synthase...
| File | emd_29653_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of TBAJ-876-bound Mycobacterium smegmatis ATP synthase rotational state 1 (Half B) | ||||||||||||
| Projections & Slices |
| ||||||||||||
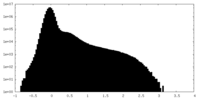
| Density Histograms |
-Half map: Cryo-EM structure of TBAJ-876-bound Mycobacterium smegmatis ATP synthase...
| File | emd_29653_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of TBAJ-876-bound Mycobacterium smegmatis ATP synthase rotational state 1 (Half A) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : TBAJ-876-bound Mycobacterium smegmatis ATP synthase rotational state 1
+Supramolecule #1: TBAJ-876-bound Mycobacterium smegmatis ATP synthase rotational state 1
+Macromolecule #1: ATP synthase subunit alpha
+Macromolecule #2: ATP synthase subunit beta
+Macromolecule #3: ATP synthase gamma chain
+Macromolecule #4: ATP synthase epsilon chain
+Macromolecule #5: ATP synthase subunit b
+Macromolecule #6: ATP synthase subunit b-delta
+Macromolecule #7: ATP synthase subunit c
+Macromolecule #8: ATP synthase subunit a
+Macromolecule #9: ADENOSINE-5'-TRIPHOSPHATE
+Macromolecule #10: MAGNESIUM ION
+Macromolecule #11: PHOSPHATE ION
+Macromolecule #12: (1R,2S)-1-(6-bromo-2-methoxyquinolin-3-yl)-2-(2,6-dimethoxypyridi...
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 44.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.1 µm / Nominal defocus min: 0.7000000000000001 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)