[English] 日本語
 Yorodumi
Yorodumi- EMDB-24790: Isoproterenol bound beta1 adrenergic receptor in complex with het... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-24790 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Isoproterenol bound beta1 adrenergic receptor in complex with heterotrimeric Gi/s chimera protein | |||||||||
 Map data Map data | Composite map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | chimera Gi/s protein / GPCR-Gi-s complex / agonist / SIGNALING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationExtra-nuclear estrogen signaling / Adenylate cyclase inhibitory pathway / Olfactory Signaling Pathway / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / sensory perception of chemical stimulus / Activation of the phototransduction cascade / mu-type opioid receptor binding / corticotropin-releasing hormone receptor 1 binding / negative regulation of synaptic transmission ...Extra-nuclear estrogen signaling / Adenylate cyclase inhibitory pathway / Olfactory Signaling Pathway / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / sensory perception of chemical stimulus / Activation of the phototransduction cascade / mu-type opioid receptor binding / corticotropin-releasing hormone receptor 1 binding / negative regulation of synaptic transmission / GTPase activating protein binding / Adrenaline,noradrenaline inhibits insulin secretion / ADP signalling through P2Y purinoceptor 12 / beta-2 adrenergic receptor binding / G alpha (i) signalling events / Activation of G protein gated Potassium channels / G-protein activation / G beta:gamma signalling through PI3Kgamma / Prostacyclin signalling through prostacyclin receptor / G beta:gamma signalling through PLC beta / ADP signalling through P2Y purinoceptor 1 / Thromboxane signalling through TP receptor / Presynaptic function of Kainate receptors / G beta:gamma signalling through CDC42 / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G alpha (12/13) signalling events / Glucagon-type ligand receptors / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Adrenaline,noradrenaline inhibits insulin secretion / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / Ca2+ pathway / Thrombin signalling through proteinase activated receptors (PARs) / G alpha (z) signalling events / Extra-nuclear estrogen signaling / G alpha (s) signalling events / G alpha (q) signalling events / neurotransmitter receptor localization to postsynaptic specialization membrane / G alpha (i) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / Vasopressin regulates renal water homeostasis via Aquaporins / D1 dopamine receptor binding / adenylate cyclase-activating adrenergic receptor signaling pathway / positive regulation of protein localization to cell cortex / T cell migration / insulin-like growth factor receptor binding / D2 dopamine receptor binding / response to prostaglandin E / ionotropic glutamate receptor binding / adenylate cyclase regulator activity / G protein-coupled serotonin receptor binding / adenylate cyclase-inhibiting serotonin receptor signaling pathway / cellular response to forskolin / regulation of mitotic spindle organization / adenylate cyclase activator activity / positive regulation of cholesterol biosynthetic process / G protein-coupled receptor binding / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / G-protein beta/gamma-subunit complex binding / adenylate cyclase-activating G protein-coupled receptor signaling pathway / photoreceptor disc membrane / GDP binding / cellular response to catecholamine stimulus / adenylate cyclase-activating dopamine receptor signaling pathway / G-protein beta-subunit binding / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / sensory perception of taste / signaling receptor complex adaptor activity / G protein activity / retina development in camera-type eye / GTPase binding / midbody / cell cortex / phospholipase C-activating G protein-coupled receptor signaling pathway / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / cell population proliferation / postsynapse / G protein-coupled receptor signaling pathway / cell division / GTPase activity / synapse / centrosome / GTP binding / protein-containing complex binding / glutamatergic synapse / magnesium ion binding / protein-containing complex / metal ion binding / nucleus / membrane / plasma membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.86 Å | |||||||||
 Authors Authors | Paknejad N / Alegre KO | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2021 Journal: Nat Struct Mol Biol / Year: 2021Title: Structural basis and mechanism of activation of two different families of G proteins by the same GPCR. Authors: Kamela O Alegre / Navid Paknejad / Minfei Su / Jian-Shu Lou / Jianyun Huang / Kelsey D Jordan / Edward T Eng / Joel R Meyerson / Richard K Hite / Xin-Yun Huang /  Abstract: The β-adrenergic receptor (β-AR) can activate two families of G proteins. When coupled to Gs, β-AR increases cardiac output, and coupling to Gi leads to decreased responsiveness in myocardial ...The β-adrenergic receptor (β-AR) can activate two families of G proteins. When coupled to Gs, β-AR increases cardiac output, and coupling to Gi leads to decreased responsiveness in myocardial infarction. By comparative structural analysis of turkey β-AR complexed with either Gi or Gs, we investigate how a single G-protein-coupled receptor simultaneously signals through two G proteins. We find that, although the critical receptor-interacting C-terminal α5-helices on Gα and Gα interact similarly with β-AR, the overall interacting modes between β-AR and G proteins vary substantially. Functional studies reveal the importance of the differing interactions and provide evidence that the activation efficacy of G proteins by β-AR is determined by the entire three-dimensional interaction surface, including intracellular loops 2 and 4 (ICL2 and ICL4). | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_24790.map.gz emd_24790.map.gz | 13.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-24790-v30.xml emd-24790-v30.xml emd-24790.xml emd-24790.xml | 21.6 KB 21.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_24790.png emd_24790.png | 95.1 KB | ||
| Filedesc metadata |  emd-24790.cif.gz emd-24790.cif.gz | 6.6 KB | ||
| Others |  emd_24790_additional_1.map.gz emd_24790_additional_1.map.gz emd_24790_additional_2.map.gz emd_24790_additional_2.map.gz emd_24790_additional_3.map.gz emd_24790_additional_3.map.gz | 5.4 MB 5.4 MB 5.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-24790 http://ftp.pdbj.org/pub/emdb/structures/EMD-24790 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24790 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24790 | HTTPS FTP |
-Validation report
| Summary document |  emd_24790_validation.pdf.gz emd_24790_validation.pdf.gz | 354.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_24790_full_validation.pdf.gz emd_24790_full_validation.pdf.gz | 353.8 KB | Display | |
| Data in XML |  emd_24790_validation.xml.gz emd_24790_validation.xml.gz | 6.6 KB | Display | |
| Data in CIF |  emd_24790_validation.cif.gz emd_24790_validation.cif.gz | 7.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24790 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24790 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24790 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24790 | HTTPS FTP |
-Related structure data
| Related structure data |  7s0gMC  7s0fC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_24790.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_24790.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Composite map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.0735 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Consensus map
| File | emd_24790_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Consensus map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Gi/s chimera focus map
| File | emd_24790_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Gi/s chimera focus map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Beta1-AR focus map
| File | emd_24790_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Beta1-AR focus map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Isoproterenol bound beta1 adrenergic receptor in complex with het...
| Entire | Name: Isoproterenol bound beta1 adrenergic receptor in complex with heterotrimeric Gi/s chimera protein |
|---|---|
| Components |
|
-Supramolecule #1: Isoproterenol bound beta1 adrenergic receptor in complex with het...
| Supramolecule | Name: Isoproterenol bound beta1 adrenergic receptor in complex with heterotrimeric Gi/s chimera protein type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 146 KDa |
-Macromolecule #1: Beta1-Adrenergic Receptor
| Macromolecule | Name: Beta1-Adrenergic Receptor / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 57.958668 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKTIIALSYI FCLVFADYKD DDDKLEVLFQ GPNIFEMLRI DEGLRLKIYK DTEGYYTIGI GHLLTKSPSL NAAKSELDKA IGRNTNGVI TKDEAEKLFN QDVDAAVRGI LRNAKLKPVY DSLDAVRRAA LINMVFQMGE TGVAGFTNSL RMLQQKRWDE A AVNLAKSR ...String: MKTIIALSYI FCLVFADYKD DDDKLEVLFQ GPNIFEMLRI DEGLRLKIYK DTEGYYTIGI GHLLTKSPSL NAAKSELDKA IGRNTNGVI TKDEAEKLFN QDVDAAVRGI LRNAKLKPVY DSLDAVRRAA LINMVFQMGE TGVAGFTNSL RMLQQKRWDE A AVNLAKSR WYNQTPNRAK RVITTFRTGT WDAYAAGAEL LSQQWEAGMS LLMALVVLLI VAGNVLVIAA IGSTQRLQTL TN LFITSLA CADLVVGLLV VPFGATLVVR GTWLWGSFLC ELWTSLDVLC VTASIETLCV IAIDRYLAIT SPFRYQSLMT RAR AKVIIC TVWAISALVS FLPIMMHWWR DEDPQALKCY QDPGCCDFVT NRAYAIASSI ISFYIPLLIM IFVYLRVYRE AKEQ IRKID RCEGRFREHK ALKTLGIIMG VFTLCWLPFF LVNIVNVFNR DLVPDWLFVF FNWLGYANSA FNPIIYCRSP DFRKA FKRL LCFPRKADRR LEVLFQGPHH HHHH |
-Macromolecule #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 37.285734 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SELDQLRQEA EQLKNQIRDA RKACADATLS QITNNIDPVG RIQMRTRRTL RGHLAKIYAM HWGTDSRLLV SASQDGKLII WDSYTTNKV HAIPLRSSWV MTCAYAPSGN YVACGGLDNI CSIYNLKTRE GNVRVSRELA GHTGYLSCCR FLDDNQIVTS S GDTTCALW ...String: SELDQLRQEA EQLKNQIRDA RKACADATLS QITNNIDPVG RIQMRTRRTL RGHLAKIYAM HWGTDSRLLV SASQDGKLII WDSYTTNKV HAIPLRSSWV MTCAYAPSGN YVACGGLDNI CSIYNLKTRE GNVRVSRELA GHTGYLSCCR FLDDNQIVTS S GDTTCALW DIETGQQTTT FTGHTGDVMS LSLAPDTRLF VSGACDASAK LWDVREGMCR QTFTGHESDI NAICFFPNGN AF ATGSDDA TCRLFDLRAD QELMTYSHDN IICGITSVSF SKSGRLLLAG YDDFNCNVWD ALKADRAGVL AGHDNRVSCL GVT DDGMAV ATGSWDSFLK IWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #3: Guanine nucleotide-binding protein G(i) subunit alpha-1,Guanine n...
| Macromolecule | Name: Guanine nucleotide-binding protein G(i) subunit alpha-1,Guanine nucleotide-binding protein G(s) subunit alpha isoforms short chimera type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 43.370332 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGSSHHHHHH SSGLEVLFQG PHMASMGCTL SAEDKAAVER SKMIDRNLRE DGEKAAREVK LLLLGAGESG KSTIVKQMKI IHEAGYSEE ECKQYKAVVY SNTIQSIIAI IRAMGRLKID FGDAARADDA RQLFVLAGAA EEGFMTAELA GVIKRLWKDS G VQACFNRS ...String: MGSSHHHHHH SSGLEVLFQG PHMASMGCTL SAEDKAAVER SKMIDRNLRE DGEKAAREVK LLLLGAGESG KSTIVKQMKI IHEAGYSEE ECKQYKAVVY SNTIQSIIAI IRAMGRLKID FGDAARADDA RQLFVLAGAA EEGFMTAELA GVIKRLWKDS G VQACFNRS REYQLNDSAA YYLNDLDRIA QPNYIPTQQD VLRTRVKTTG IVETHFTFKD LHFKMFDVGA QRSERKKWIH CF EGVTAII FCVALSDYDL VLAEDEEMNR MHESMKLFDS ICNNKWFTDT SIILFLNKKD LFEEKIKKSP LTICYPEYAG SNT YEEAAA YIQCQFEDLN KRKDTKEIYT HFTCATDTKN VQFVFDAVTD VIQRMHLRQY ELL UniProtKB: Guanine nucleotide-binding protein G(i) subunit alpha-1, Guanine nucleotide-binding protein G(s) subunit alpha isoforms short |
-Macromolecule #4: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 7.845078 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFSAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #5: ISOPRENALINE
| Macromolecule | Name: ISOPRENALINE / type: ligand / ID: 5 / Number of copies: 1 / Formula: 5FW |
|---|---|
| Molecular weight | Theoretical: 211.258 Da |
| Chemical component information | 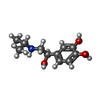 ChemComp-5FW: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Number real images: 3284 / Average electron dose: 71.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.86 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 168662 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD / Software - Name: cryoSPARC (ver. v2) / Details: ab initio |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD / Software - Name: cryoSPARC (ver. v2) / Details: non-uniform refinement |
 Movie
Movie Controller
Controller























 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)













































