[English] 日本語
 Yorodumi
Yorodumi- EMDB-24380: Composite map of axonemal microtubule doublet in 48 nm repeat fro... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-24380 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Composite map of axonemal microtubule doublet in 48 nm repeat from Tetrahymena thermophila | ||||||||||||
 Map data Map data | Composite structure of axonemal microtubule doublet in 48 nm repeat from Tetrahymena thermophila | ||||||||||||
 Sample Sample |
| ||||||||||||
| Biological species |  | ||||||||||||
| Method | subtomogram averaging / cryo EM / Resolution: 12.0 Å | ||||||||||||
 Authors Authors | Li S / Fernandez JJ / Fabritius A / Agard DA / Winey M | ||||||||||||
| Funding support |  United States, United States,  Spain, 3 items Spain, 3 items
| ||||||||||||
 Citation Citation |  Journal: Life Sci Alliance / Year: 2022 Journal: Life Sci Alliance / Year: 2022Title: Electron cryo-tomography structure of axonemal doublet microtubule from . Authors: Sam Li / Jose-Jesus Fernandez / Amy S Fabritius / David A Agard / Mark Winey /   Abstract: Doublet microtubules (DMTs) provide a scaffold for axoneme assembly in motile cilia. Aside from α/β tubulins, the DMT comprises a large number of non-tubulin proteins in the luminal wall of DMTs, ...Doublet microtubules (DMTs) provide a scaffold for axoneme assembly in motile cilia. Aside from α/β tubulins, the DMT comprises a large number of non-tubulin proteins in the luminal wall of DMTs, collectively named the microtubule inner proteins (MIPs). We used cryoET to study axoneme DMT isolated from We present the structures of DMT at nanometer and sub-nanometer resolution. The structures confirm that MIP RIB72A/B binds to the luminal wall of DMT by multiple DM10 domains. We found FAP115, an MIP-containing multiple EF-hand domains, located at the interface of four-tubulin dimers in the lumen of A-tubule. It contacts both lateral and longitudinal tubulin interfaces and playing a critical role in DMT stability. We observed substantial structure heterogeneity in DMT in an knockout strain, showing extensive structural defects beyond the FAP115-binding site. The defects propagate along the axoneme. Finally, by comparing DMT structures from and , we have identified a number of conserved MIPs as well as MIPs that are unique to each organism. This conservation and diversity of the DMT structures might be linked to their specific functions. Our work provides structural insights essential for understanding the roles of MIPs during motile cilium assembly and function, as well as their relationships to human ciliopathies. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_24380.map.gz emd_24380.map.gz | 57.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-24380-v30.xml emd-24380-v30.xml emd-24380.xml emd-24380.xml | 11.2 KB 11.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_24380.png emd_24380.png | 78.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-24380 http://ftp.pdbj.org/pub/emdb/structures/EMD-24380 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24380 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24380 | HTTPS FTP |
-Validation report
| Summary document |  emd_24380_validation.pdf.gz emd_24380_validation.pdf.gz | 336.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_24380_full_validation.pdf.gz emd_24380_full_validation.pdf.gz | 336.2 KB | Display | |
| Data in XML |  emd_24380_validation.xml.gz emd_24380_validation.xml.gz | 4.7 KB | Display | |
| Data in CIF |  emd_24380_validation.cif.gz emd_24380_validation.cif.gz | 5.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24380 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24380 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24380 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24380 | HTTPS FTP |
-Related structure data
| Related structure data | C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_24380.map.gz / Format: CCP4 / Size: 80.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_24380.map.gz / Format: CCP4 / Size: 80.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Composite structure of axonemal microtubule doublet in 48 nm repeat from Tetrahymena thermophila | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.65 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
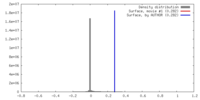
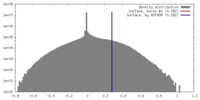
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Axonemal microtubule doublet from Tetrahymena thermophile
| Entire | Name: Axonemal microtubule doublet from Tetrahymena thermophile |
|---|---|
| Components |
|
-Supramolecule #1: Axonemal microtubule doublet from Tetrahymena thermophile
| Supramolecule | Name: Axonemal microtubule doublet from Tetrahymena thermophile type: organelle_or_cellular_component / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: Quantifoil R2/2 / Material: COPPER/RHODIUM / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 70.0 K / Max: 70.0 K |
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average exposure time: 0.36 sec. / Average electron dose: 1.31 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Calibrated defocus max: 4.0 µm / Calibrated defocus min: 2.0 µm / Calibrated magnification: 18868 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 3.0 µm / Nominal magnification: 33000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 12.0 Å / Resolution method: FSC 0.143 CUT-OFF Details: This is a composite map. The resolution is not directly assessed by the FSC method. It is taken from the lowest value from one of its component maps. Number subtomograms used: 5855 |
|---|---|
| Extraction | Number tomograms: 49 / Number images used: 18762 |
| Final angle assignment | Type: OTHER |
 Movie
Movie Controller
Controller






















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)