[English] 日本語
 Yorodumi
Yorodumi- EMDB-24131: Continuous movement of the central protuberance domain of 50S rib... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-24131 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Continuous movement of the central protuberance domain of 50S ribosome. | |||||||||
 Map data Map data | Continuous movement of the central protuberance domain of 50S ribosome. | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 8.0 Å | |||||||||
 Authors Authors | Chen M / Ludtke SJ | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Methods / Year: 2021 Journal: Nat Methods / Year: 2021Title: Deep learning-based mixed-dimensional Gaussian mixture model for characterizing variability in cryo-EM. Authors: Muyuan Chen / Steven J Ludtke /  Abstract: Structural flexibility and/or dynamic interactions with other molecules is a critical aspect of protein function. Cryogenic electron microscopy (cryo-EM) provides direct visualization of individual ...Structural flexibility and/or dynamic interactions with other molecules is a critical aspect of protein function. Cryogenic electron microscopy (cryo-EM) provides direct visualization of individual macromolecules sampling different conformational and compositional states. While numerous methods are available for computational classification of discrete states, characterization of continuous conformational changes or large numbers of discrete state without human supervision remains challenging. Here we present e2gmm, a machine learning algorithm to determine a conformational landscape for proteins or complexes using a three-dimensional Gaussian mixture model mapped onto two-dimensional particle images in known orientations. Using a deep neural network architecture, e2gmm can automatically resolve the structural heterogeneity within the protein complex and map particles onto a small latent space describing conformational and compositional changes. This system presents a more intuitive and flexible representation than other manifold methods currently in use. We demonstrate this method on both simulated data and three biological systems to explore compositional and conformational changes at a range of scales. The software is distributed as part of EMAN2. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_24131.map.gz emd_24131.map.gz | 7.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-24131-v30.xml emd-24131-v30.xml emd-24131.xml emd-24131.xml | 18.5 KB 18.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_24131.png emd_24131.png | 57.3 KB | ||
| Others |  emd_24131_additional_1.map.gz emd_24131_additional_1.map.gz emd_24131_additional_2.map.gz emd_24131_additional_2.map.gz emd_24131_additional_3.map.gz emd_24131_additional_3.map.gz emd_24131_additional_4.map.gz emd_24131_additional_4.map.gz emd_24131_additional_5.map.gz emd_24131_additional_5.map.gz | 7.4 MB 7.4 MB 7.4 MB 7.4 MB 7.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-24131 http://ftp.pdbj.org/pub/emdb/structures/EMD-24131 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24131 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24131 | HTTPS FTP |
-Validation report
| Summary document |  emd_24131_validation.pdf.gz emd_24131_validation.pdf.gz | 358 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_24131_full_validation.pdf.gz emd_24131_full_validation.pdf.gz | 357.5 KB | Display | |
| Data in XML |  emd_24131_validation.xml.gz emd_24131_validation.xml.gz | 5.7 KB | Display | |
| Data in CIF |  emd_24131_validation.cif.gz emd_24131_validation.cif.gz | 6.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24131 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24131 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24131 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24131 | HTTPS FTP |
-Related structure data
| Related structure data | C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_24131.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_24131.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Continuous movement of the central protuberance domain of 50S ribosome. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.62 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
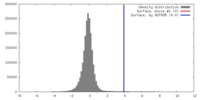
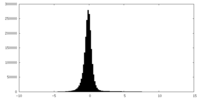
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Continuous movement of the central protuberance domain of...
| File | emd_24131_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Continuous movement of the central protuberance domain of 50S ribosome. Movie frame 1. | ||||||||||||
| Projections & Slices |
| ||||||||||||
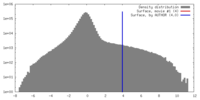
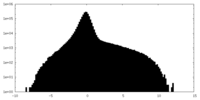
| Density Histograms |
-Additional map: Continuous movement of the central protuberance domain of...
| File | emd_24131_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Continuous movement of the central protuberance domain of 50S ribosome. Movie frame 2. | ||||||||||||
| Projections & Slices |
| ||||||||||||
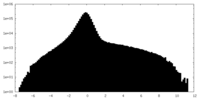
| Density Histograms |
-Additional map: Continuous movement of the central protuberance domain of...
| File | emd_24131_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Continuous movement of the central protuberance domain of 50S ribosome. Movie frame 3. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Continuous movement of the central protuberance domain of...
| File | emd_24131_additional_4.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Continuous movement of the central protuberance domain of 50S ribosome. Movie frame 4. | ||||||||||||
| Projections & Slices |
| ||||||||||||
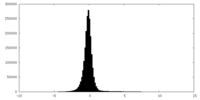
| Density Histograms |
-Additional map: Continuous movement of the central protuberance domain of...
| File | emd_24131_additional_5.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Continuous movement of the central protuberance domain of 50S ribosome. Movie frame 5. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : bL17-depleted large ribosomal subunit assembly intermediate
| Entire | Name: bL17-depleted large ribosomal subunit assembly intermediate |
|---|---|
| Components |
|
-Supramolecule #1: bL17-depleted large ribosomal subunit assembly intermediate
| Supramolecule | Name: bL17-depleted large ribosomal subunit assembly intermediate type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 34.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: EMDB MAP EMDB ID: |
|---|---|
| Final reconstruction | Number classes used: 5 / Resolution.type: BY AUTHOR / Resolution: 8.0 Å / Resolution method: OTHER Details: 2000 particles are used for each frame of the movement. The maps are filtered to 8A for display. Number images used: 2000 |
| Initial angle assignment | Type: PROJECTION MATCHING |
| Final angle assignment | Type: PROJECTION MATCHING / Software - Name: EMAN (ver. 2.91) |
 Movie
Movie Controller
Controller



















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)