+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-21360 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Type I-F CRISPR-Csy complex with its inhibitor AcrF6 | ||||||||||||||||||
 Map data Map data | Type I-F CRISPR-Csy complex with its inhibitor AcrF6 | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | CRISPR / Type I-F / Csy / AcrF6 / anti-CRISPR / RNA BINDING PROTEIN-RNA-INHIBITOR complex | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationmaintenance of CRISPR repeat elements / endonuclease activity / defense response to virus / Hydrolases; Acting on ester bonds / RNA binding Similarity search - Function | ||||||||||||||||||
| Biological species |  | ||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.15 Å | ||||||||||||||||||
 Authors Authors | Zhang K / Li S | ||||||||||||||||||
| Funding support |  United States, United States,  China, 5 items China, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2020 Journal: Proc Natl Acad Sci U S A / Year: 2020Title: Inhibition mechanisms of AcrF9, AcrF8, and AcrF6 against type I-F CRISPR-Cas complex revealed by cryo-EM. Authors: Kaiming Zhang / Shuo Wang / Shanshan Li / Yuwei Zhu / Grigore D Pintilie / Tung-Chung Mou / Michael F Schmid / Zhiwei Huang / Wah Chiu /   Abstract: Prokaryotes and viruses have fought a long battle against each other. Prokaryotes use CRISPR-Cas-mediated adaptive immunity, while conversely, viruses evolve multiple anti-CRISPR (Acr) proteins to ...Prokaryotes and viruses have fought a long battle against each other. Prokaryotes use CRISPR-Cas-mediated adaptive immunity, while conversely, viruses evolve multiple anti-CRISPR (Acr) proteins to defeat these CRISPR-Cas systems. The type I-F CRISPR-Cas system in requires the crRNA-guided surveillance complex (Csy complex) to recognize the invading DNA. Although some Acr proteins against the Csy complex have been reported, other relevant Acr proteins still need studies to understand their mechanisms. Here, we obtain three structures of previously unresolved Acr proteins (AcrF9, AcrF8, and AcrF6) bound to the Csy complex using electron cryo-microscopy (cryo-EM), with resolution at 2.57 Å, 3.42 Å, and 3.15 Å, respectively. The 2.57-Å structure reveals fine details for each molecular component within the Csy complex as well as the direct and water-mediated interactions between proteins and CRISPR RNA (crRNA). Our structures also show unambiguously how these Acr proteins bind differently to the Csy complex. AcrF9 binds to key DNA-binding sites on the Csy spiral backbone. AcrF6 binds at the junction between Cas7.6f and Cas8f, which is critical for DNA duplex splitting. AcrF8 binds to a distinct position on the Csy spiral backbone and forms interactions with crRNA, which has not been seen in other Acr proteins against the Csy complex. Our structure-guided mutagenesis and biochemistry experiments further support the anti-CRISPR mechanisms of these Acr proteins. Our findings support the convergent consequence of inhibiting degradation of invading DNA by these Acr proteins, albeit with different modes of interactions with the type I-F CRISPR-Cas system. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_21360.map.gz emd_21360.map.gz | 11 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-21360-v30.xml emd-21360-v30.xml emd-21360.xml emd-21360.xml | 20.3 KB 20.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_21360.png emd_21360.png | 193.2 KB | ||
| Filedesc metadata |  emd-21360.cif.gz emd-21360.cif.gz | 6.8 KB | ||
| Others |  emd_21360_additional.map.gz emd_21360_additional.map.gz | 21.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-21360 http://ftp.pdbj.org/pub/emdb/structures/EMD-21360 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21360 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21360 | HTTPS FTP |
-Related structure data
| Related structure data |  6vqxMC  6vqvC  6vqwC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_21360.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_21360.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Type I-F CRISPR-Csy complex with its inhibitor AcrF6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
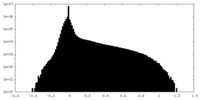
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
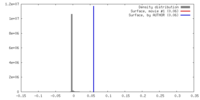
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Unmasked map
| File | emd_21360_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unmasked map | ||||||||||||
| Projections & Slices |
| ||||||||||||
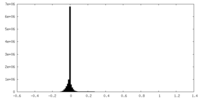
| Density Histograms |
- Sample components
Sample components
-Entire : Type I-F CRISPR-Csy complex with its inhibitor AcrF6
| Entire | Name: Type I-F CRISPR-Csy complex with its inhibitor AcrF6 |
|---|---|
| Components |
|
-Supramolecule #1: Type I-F CRISPR-Csy complex with its inhibitor AcrF6
| Supramolecule | Name: Type I-F CRISPR-Csy complex with its inhibitor AcrF6 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 350 KDa |
-Macromolecule #1: AcrF6
| Macromolecule | Name: AcrF6 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 10.787753 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKVPAFFAAN ILTIEQIIEA INNDGSAMTS APEIAGYYAW DAATDALESE NDLEQLTEDD FVAHLEVLEE RGAKIDRDAA IAVALQFQA AAVNDLHSGD E UniProtKB: UNIPROTKB: A0A5D4V3F2 |
-Macromolecule #2: CRISPR-associated protein Csy1
| Macromolecule | Name: CRISPR-associated protein Csy1 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 49.194168 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTSPLPTPTW QELRQFIESF IQERLQGKLD KLQPDEDDKR QTLLATHRRE AWLADAARRV GQLQLVTHTL KPIHPDARGS NLHSLPQAP GQPGLAGSHE LGDRLVSDVV GNAAALDVFK FLSLQYQGKN LLNWLTEDSA EALQALSDNA EQAREWRQAF I GITTVKGA ...String: MTSPLPTPTW QELRQFIESF IQERLQGKLD KLQPDEDDKR QTLLATHRRE AWLADAARRV GQLQLVTHTL KPIHPDARGS NLHSLPQAP GQPGLAGSHE LGDRLVSDVV GNAAALDVFK FLSLQYQGKN LLNWLTEDSA EALQALSDNA EQAREWRQAF I GITTVKGA PASHSLAKQL YFPLPGSGYH LLAPLFPTSL VHHVHALLRE ARFGDAAKAA REARSRQESW PHGFSEYPNL AI QKFGGTK PQNISQLNNE RRGENWLLPS LPPNWQRQNV NAPMRHSSVF EHDFGRTPEV SRLTRTLQRF LAKTVHNNLA IRQ RRAQLV AQICDEALQY AARLRELEPG WSATPGCQLH DAEQLWLDPL RAQTDETFLQ RRLRGDWPAE VGNRFANWLN RAVS SDSQI LGSPEAAQWS QELSKELTMF KEILEDERD UniProtKB: CRISPR-associated protein Csy1 |
-Macromolecule #3: Type I-F CRISPR-associated protein Csy2
| Macromolecule | Name: Type I-F CRISPR-associated protein Csy2 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 36.244074 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSVTDPEALL LLPRLSIQNA NAISSPLTWG FPSPGAFTGF VHALQRRVGI SLDIELDGVG IVCHRFEAQI SQPAGKRTKV FNLTRNPLN RDGSTAAIVE EGRAHLEVSL LLGVHGDGLD DHPAQEIARQ VQEQAGAMRL AGGSILPWCN ERFPAPNAEL L MLGGSDEQ ...String: MSVTDPEALL LLPRLSIQNA NAISSPLTWG FPSPGAFTGF VHALQRRVGI SLDIELDGVG IVCHRFEAQI SQPAGKRTKV FNLTRNPLN RDGSTAAIVE EGRAHLEVSL LLGVHGDGLD DHPAQEIARQ VQEQAGAMRL AGGSILPWCN ERFPAPNAEL L MLGGSDEQ RRKNQRRLTR RLLPGFALVS REALLQQHLE TLRTTLPEAT TLDALLDLCR INFEPPATSS EEEASPPDAA WQ VRDKPGW LVPIPAGYNA LSPLYLPGEV RNARDRETPL RFVENLFGLG EWLSPHRVAA LSDLLWYHHA EPDKGLYRWS TPR FVEHAI A UniProtKB: Uncharacterized protein |
-Macromolecule #4: CRISPR-associated protein Csy3
| Macromolecule | Name: CRISPR-associated protein Csy3 / type: protein_or_peptide / ID: 4 / Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 39.778594 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKSSHHHHHH ENLYFQSNAS KPILSTASVL AFERKLDPSD ALMSAGAWAQ RDASQEWPAV TVREKSVRGT ISNRLKTKDR DPAKLDASI QSPNLQTVDV ANLPSDADTL KVRFTLRVLG GAGTPSACND AAYRDKLLQT VATYVNDQGF AELARRYAHN L ANARFLWR ...String: MKSSHHHHHH ENLYFQSNAS KPILSTASVL AFERKLDPSD ALMSAGAWAQ RDASQEWPAV TVREKSVRGT ISNRLKTKDR DPAKLDASI QSPNLQTVDV ANLPSDADTL KVRFTLRVLG GAGTPSACND AAYRDKLLQT VATYVNDQGF AELARRYAHN L ANARFLWR NRVGAEAVEV RINHIRQGEV ARAWRFDALA IGLRDFKADA ELDALAELIA SGLSGSGHVL LEVVAFARIG DG QEVFPSQ ELILDKGDKK GQKSKTLYSV RDAAAIHSQK IGNALRTIDT WYPDEDGLGP IAVEPYGSVT SQGKAYRQPK QKL DFYTLL DNWVLRDEAP AVEQQHYVIA NLIRGGVFGE AEEK UniProtKB: CRISPR type I-F/YPEST-associated protein Csy3 |
-Macromolecule #5: CRISPR-associated endonuclease Cas6/Csy4
| Macromolecule | Name: CRISPR-associated endonuclease Cas6/Csy4 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO / EC number: Hydrolases; Acting on ester bonds |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 21.427504 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MDHYLDIRLR PDPEFPPAQL MSVLFGKLHQ ALVAQGGDRI GVSFPDLDES RSRLGERLRI HASADDLRAL LARPWLEGLR DHLQFGEPA VVPHPTPYRQ VSRVQAKSNP ERLRRRLMRR HDLSEEEARK RIPDTVARAL DLPFVTLRSQ STGQHFRLFI R HGPLQVTA EEGGFTCYGL SKGGFVPWF UniProtKB: CRISPR-associated endonuclease Cas6/Csy4 |
-Macromolecule #6: CrRNA (60-MER)
| Macromolecule | Name: CrRNA (60-MER) / type: rna / ID: 6 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 19.249404 KDa |
| Sequence | String: CUAAGAAAUU CACGGCGGGC UUGAUGUCCG CGUCUACCUG GUUCACUGCC GUAUAGGCAG |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.4 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Grid | Details: unspecified |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Number real images: 1956 / Average exposure time: 6.0 sec. / Average electron dose: 7.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 3.15 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION (ver. 3.0.2) / Number images used: 56455 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)