[English] 日本語
 Yorodumi
Yorodumi- PDB-1ek6: STRUCTURE OF HUMAN UDP-GALACTOSE 4-EPIMERASE COMPLEXED WITH NADH ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1ek6 | ||||||
|---|---|---|---|---|---|---|---|
| Title | STRUCTURE OF HUMAN UDP-GALACTOSE 4-EPIMERASE COMPLEXED WITH NADH AND UDP-GLUCOSE | ||||||
 Components Components | UDP-GALACTOSE 4-EPIMERASE | ||||||
 Keywords Keywords | ISOMERASE / Epimerase / Short-Chain Dehydrogenase / Galactosemia | ||||||
| Function / homology |  Function and homology information Function and homology informationDefective GALE causes EDG / UDP-N-acetylglucosamine 4-epimerase activity / UDP-N-acetylglucosamine 4-epimerase / galactose catabolic process / UDP-glucose 4-epimerase / UDP-glucose 4-epimerase activity / galactose catabolic process via UDP-galactose, Leloir pathway / Galactose catabolism / protein homodimerization activity / identical protein binding / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / Resolution: 1.5 Å SYNCHROTRON / Resolution: 1.5 Å | ||||||
 Authors Authors | Thoden, J.B. / Wohlers, T.M. / Fridovich-Keil, J.L. / Holden, H.M. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2000 Journal: Biochemistry / Year: 2000Title: Crystallographic evidence for Tyr 157 functioning as the active site base in human UDP-galactose 4-epimerase. Authors: Thoden, J.B. / Wohlers, T.M. / Fridovich-Keil, J.L. / Holden, H.M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1ek6.cif.gz 1ek6.cif.gz | 174.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1ek6.ent.gz pdb1ek6.ent.gz | 135 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1ek6.json.gz 1ek6.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ek/1ek6 https://data.pdbj.org/pub/pdb/validation_reports/ek/1ek6 ftp://data.pdbj.org/pub/pdb/validation_reports/ek/1ek6 ftp://data.pdbj.org/pub/pdb/validation_reports/ek/1ek6 | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Details | homodimer constructed from chains A & B |
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 38324.605 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Tissue: FORESKIN / Production host: Homo sapiens (human) / Tissue: FORESKIN / Production host:  |
|---|
-Non-polymers , 5 types, 908 molecules 

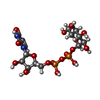





| #2: Chemical | | #3: Chemical | #4: Chemical | #5: Chemical | #6: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.02 Å3/Da / Density % sol: 39.02 % | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 273 K / Method: batch / pH: 6 Details: PEG 3400, magnesium chloride, MES, NADH, UDP-glucose, sodium chloride, pH 6.0, BATCH, temperature 273.0K | ||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Method: batch method | ||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 19-ID / Wavelength: 0.7 / Beamline: 19-ID / Wavelength: 0.7 |
| Detector | Type: SBC-2 / Detector: CCD / Date: Dec 21, 1999 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.7 Å / Relative weight: 1 |
| Reflection | Resolution: 1.5→30 Å / Num. all: 96156 / Num. obs: 96156 / % possible obs: 96.1 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 5.5 % / Rmerge(I) obs: 0.05 / Net I/σ(I): 29.9 |
| Reflection shell | Resolution: 1.5→1.55 Å / Redundancy: 3.6 % / Rmerge(I) obs: 0.286 / Num. unique all: 8401 / % possible all: 84.7 |
| Reflection | *PLUS |
| Reflection shell | *PLUS % possible obs: 84.7 % / Num. unique obs: 8401 / Mean I/σ(I) obs: 2.9 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 1.5→30 Å / σ(F): 0 / σ(I): 0 / Stereochemistry target values: Engh & Huber
| |||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.5→30 Å
| |||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||
| Software | *PLUS Name: TNT / Version: 5E / Classification: refinement | |||||||||||||||||||||||||
| Refinement | *PLUS Num. reflection obs: 96012 / σ(F): 0 / Rfactor all: 0.169 | |||||||||||||||||||||||||
| Solvent computation | *PLUS | |||||||||||||||||||||||||
| Displacement parameters | *PLUS | |||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller













 PDBj
PDBj






