[English] 日本語
 Yorodumi
Yorodumi- PDB-1bp4: USE OF PAPAIN AS A MODEL FOR THE STRUCTURE-BASED DESIGN OF CATHEP... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1bp4 | ||||||
|---|---|---|---|---|---|---|---|
| Title | USE OF PAPAIN AS A MODEL FOR THE STRUCTURE-BASED DESIGN OF CATHEPSIN K INHIBITORS. CRYSTAL STRUCTURES OF TWO PAPAIN INHIBITOR COMPLEXES DEMONSTRATE BINDING TO S'-SUBSITES. | ||||||
 Components Components | PAPAIN | ||||||
 Keywords Keywords | HYDROLASE / SULFHYDRYL PROTEINASE | ||||||
| Function / homology |  Function and homology information Function and homology informationpapain / serpin family protein binding / cysteine-type peptidase activity / proteolysis Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 2.2 Å MOLECULAR REPLACEMENT / Resolution: 2.2 Å | ||||||
 Authors Authors | Lalonde, J.M. / Zhao, B. / Smith, W.W. / Janson, C.A. / Desjarlais, R.L. / Tomaszek, T.A. / Carr, T.J. / Thompson, S.K. / Yamashita, D.S. / Veber, D.F. / Abdel-Mequid, S.S. | ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 1998 Journal: J.Med.Chem. / Year: 1998Title: Use of papain as a model for the structure-based design of cathepsin K inhibitors: crystal structures of two papain-inhibitor complexes demonstrate binding to S'-subsites. Authors: LaLonde, J.M. / Zhao, B. / Smith, W.W. / Janson, C.A. / DesJarlais, R.L. / Tomaszek, T.A. / Carr, T.J. / Thompson, S.K. / Oh, H.J. / Yamashita, D.S. / Veber, D.F. / Abdel-Meguid, S.S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1bp4.cif.gz 1bp4.cif.gz | 58.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1bp4.ent.gz pdb1bp4.ent.gz | 41.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1bp4.json.gz 1bp4.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/bp/1bp4 https://data.pdbj.org/pub/pdb/validation_reports/bp/1bp4 ftp://data.pdbj.org/pub/pdb/validation_reports/bp/1bp4 ftp://data.pdbj.org/pub/pdb/validation_reports/bp/1bp4 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1bqiC 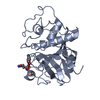 1pipS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 23449.346 Da / Num. of mol.: 1 / Fragment: NON / Source method: isolated from a natural source Details: ALDEHYDE INHIBITOR COVALENTLY BOUND TO ACTIVE SITE CYS 25 AS A HEMIMERCAPTAL. BOND OCCURS BETWEEN CYS 25-SG AND ALD 213-C22 Source: (natural)  |
|---|---|
| #2: Chemical | ChemComp-ALD / |
| #3: Water | ChemComp-HOH / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 6 X-RAY DIFFRACTION / Number of used crystals: 6 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.34 Å3/Da / Density % sol: 63 % | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | pH: 8.5 / Details: pH 8.5 | ||||||||||||||||||||
| Crystal | *PLUS | ||||||||||||||||||||
| Crystal grow | *PLUS Method: vapor diffusion, hanging drop | ||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 287 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: SIEMENS / Wavelength: 1.5418 ROTATING ANODE / Type: SIEMENS / Wavelength: 1.5418 |
| Detector | Type: SIEMENS / Detector: AREA DETECTOR / Date: Jun 1, 1995 |
| Radiation | Monochromator: MONOCHROMATOR / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 2.2→10 Å / Num. obs: 14458 / % possible obs: 95 % / Observed criterion σ(I): 2 / Redundancy: 4.3 % / Rmerge(I) obs: 0.104 / Rsym value: 0.088 / Net I/σ(I): 7.9 |
| Reflection shell | Resolution: 2.2→2.34 Å / Redundancy: 2.3 % / Mean I/σ(I) obs: 1.8 / Rsym value: 0.27 / % possible all: 60 |
| Reflection | *PLUS Num. measured all: 62560 / Rmerge(I) obs: 0.0878 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1PIP Resolution: 2.2→10 Å / Data cutoff high absF: 100000 / Data cutoff low absF: 0.1 / Isotropic thermal model: RESTRAINED / σ(F): 2 Details: DISORDERED SIDE-CHAINS HAVE OCCUPANCIES SET TO ZERO IN COORDINATE FILE, RESIDUES 59,73,78,84,93,94,98,111,114,129,145
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 22.7 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.2→10 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.2→2.3 Å / Total num. of bins used: 8
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Version: 3.1 / Classification: refinement X-PLOR / Version: 3.1 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Rfactor obs: 0.19 / Rfactor Rfree: 0.24 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller











 PDBj
PDBj