[English] 日本語
 Yorodumi
Yorodumi- EMDB-12234: C. thermophilum Pyruvate Dehydrogenase Complex Core from native c... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12234 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | C. thermophilum Pyruvate Dehydrogenase Complex Core from native cell extracts | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Biological species |  Chaetomium thermophilum var. thermophilum DSM 1495 (fungus) Chaetomium thermophilum var. thermophilum DSM 1495 (fungus) | |||||||||
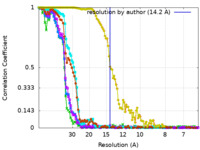
| Method | single particle reconstruction / negative staining / Resolution: 14.2 Å | |||||||||
 Authors Authors | Kyrilis FL / Semchonok DA / Skalidis I / Tueting C / Hamdi F / O'Reilly FJ / Rappsilber J / Kastritis PL | |||||||||
 Citation Citation |  Journal: Cell Rep / Year: 2021 Journal: Cell Rep / Year: 2021Title: Integrative structure of a 10-megadalton eukaryotic pyruvate dehydrogenase complex from native cell extracts. Authors: Fotis L Kyrilis / Dmitry A Semchonok / Ioannis Skalidis / Christian Tüting / Farzad Hamdi / Francis J O'Reilly / Juri Rappsilber / Panagiotis L Kastritis /   Abstract: The pyruvate dehydrogenase complex (PDHc) is a giant enzymatic assembly involved in pyruvate oxidation. PDHc components have been characterized in isolation, but the complex's quaternary structure ...The pyruvate dehydrogenase complex (PDHc) is a giant enzymatic assembly involved in pyruvate oxidation. PDHc components have been characterized in isolation, but the complex's quaternary structure has remained elusive due to sheer size, heterogeneity, and plasticity. Here, we identify fully assembled Chaetomium thermophilum α-keto acid dehydrogenase complexes in native cell extracts and characterize their domain arrangements utilizing mass spectrometry, activity assays, crosslinking, electron microscopy (EM), and computational modeling. We report the cryo-EM structure of the PDHc core and observe unique features of the previously unknown native state. The asymmetric reconstruction of the 10-MDa PDHc resolves spatial proximity of its components, agrees with stoichiometric data (60 E2p:12 E3BP:∼20 E1p: ≤ 12 E3), and proposes a minimum reaction path among component enzymes. The PDHc shows the presence of a dynamic pyruvate oxidation compartment, organized by core and peripheral protein species. Our data provide a framework for further understanding PDHc and α-keto acid dehydrogenase complex structure and function. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
-Validation report
| Summary document |  emd_12234_validation.pdf.gz emd_12234_validation.pdf.gz | 261.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_12234_full_validation.pdf.gz emd_12234_full_validation.pdf.gz | 260.6 KB | Display | |
| Data in XML |  emd_12234_validation.xml.gz emd_12234_validation.xml.gz | 14.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12234 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12234 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12234 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12234 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_12234.map.gz / Format: CCP4 / Size: 59.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12234.map.gz / Format: CCP4 / Size: 59.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
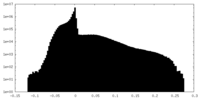
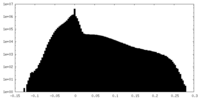
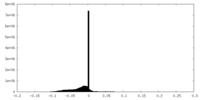
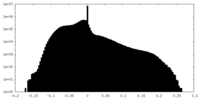
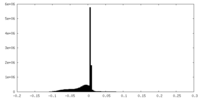
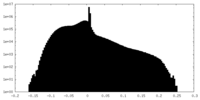
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 4.03 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
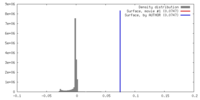
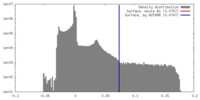
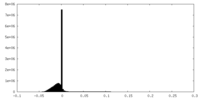
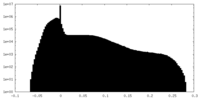
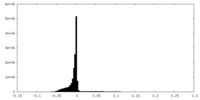
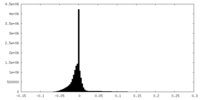
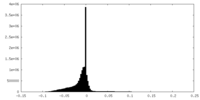
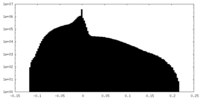
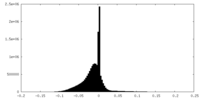
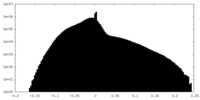
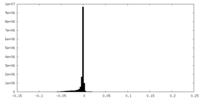
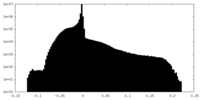
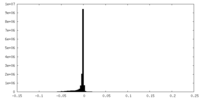
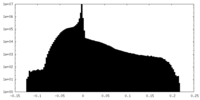
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
+Additional map: #18
+Additional map: #6
+Additional map: #5
+Additional map: #8
+Additional map: #7
+Additional map: #10
+Additional map: #9
+Additional map: #2
+Additional map: #1
+Additional map: #13
+Additional map: #12
+Additional map: #11
+Additional map: #15
+Additional map: #14
+Additional map: #17
+Additional map: #16
+Additional map: #4
+Additional map: #3
+Half map: #2
+Half map: #1
- Sample components
Sample components
-Entire : Native core of the Pyruvate Dehydrogenase Complex from C. thermophilum
| Entire | Name: Native core of the Pyruvate Dehydrogenase Complex from C. thermophilum |
|---|---|
| Components |
|
-Supramolecule #1: Native core of the Pyruvate Dehydrogenase Complex from C. thermophilum
| Supramolecule | Name: Native core of the Pyruvate Dehydrogenase Complex from C. thermophilum type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Chaetomium thermophilum var. thermophilum DSM 1495 (fungus) Chaetomium thermophilum var. thermophilum DSM 1495 (fungus) |
| Molecular weight | Theoretical: 3 MDa |
-Macromolecule #1: Dihydrolipoyl transacylase, Pyruvate Dehydrogenase Complex E2 com...
| Macromolecule | Name: Dihydrolipoyl transacylase, Pyruvate Dehydrogenase Complex E2 component type: protein_or_peptide / ID: 1 / Enantiomer: LEVO / EC number: dihydrolipoyllysine-residue acetyltransferase |
|---|---|
| Source (natural) | Organism:  Chaetomium thermophilum var. thermophilum DSM 1495 (fungus) Chaetomium thermophilum var. thermophilum DSM 1495 (fungus) |
| Sequence | String: MLAQVLRRQA LQHVRLARAA APSLTRWYAS YPPHTIVKMP ALSPTMTSGN IGAWQKKPGD AITPGEVLVE IETDKAQMDF EFQEEGVLAK ILKETGEKDV AVGSPIAVLV EEGTDINAFQ NFTLEDAGGD AAAPAAPAKE ELAKAETAPT PASTSAPEPE ETTSTGKLEP ...String: MLAQVLRRQA LQHVRLARAA APSLTRWYAS YPPHTIVKMP ALSPTMTSGN IGAWQKKPGD AITPGEVLVE IETDKAQMDF EFQEEGVLAK ILKETGEKDV AVGSPIAVLV EEGTDINAFQ NFTLEDAGGD AAAPAAPAKE ELAKAETAPT PASTSAPEPE ETTSTGKLEP ALDREPNVSF AAKKLAHELD VPLKALKGTG PGGKITEEDV KKAASAPAAA AAAPGAAYQD IPISNMRKTI ATRLKESVSE NPHFFVTSEL SVSKLLKLRQ ALNSSAEGRY KLSVNDFLIK AIAVACKRVP AVNSSWRDGV IRQFDTVDVS VAVATPTGLI TPIVKGVEAK GLETISATVK ELAKKARDGK LKPEDYQGGT ISISNMGMNP AVERFTAIIN PPQAAILAVG TTKKVAVPVE NEDGTTGVEW DDQIVVTASF DHKVVDGAVG AEWMRELKKV VENPLELLL |
-Experimental details
-Structure determination
| Method | negative staining |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.02 mg/mL |
|---|---|
| Buffer | pH: 7.4 / Component - Concentration: 200.0 mM / Component - Formula: NH4CH2COOH / Component - Name: Ammonium acetate |
| Staining | Type: NEGATIVE / Material: Uranyl Acetate |
| Grid | Model: C-flat / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 10.0 nm / Pretreatment - Type: GLOW DISCHARGE |
| Details | fractionated native cell extract |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Temperature | Min: 77.15 K / Max: 120.0 K |
| Details | Coma Free kept better than 200nm in EPU |
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: INTEGRATING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Digitization - Sampling interval: 14.0 µm / Number grids imaged: 1 / Number real images: 1926 / Average electron dose: 30.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Calibrated magnification: 34739 / Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 36000 |
| Sample stage | Specimen holder model: OTHER / Cooling holder cryogen: NITROGEN |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: A |
|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)