[English] 日本語
 Yorodumi
Yorodumi- EMDB-11631: Aquifex aeolicus lumazine synthase-derived nucleocapsid variant N... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-11631 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Aquifex aeolicus lumazine synthase-derived nucleocapsid variant NC-1 (120-mer) | |||||||||
 Map data Map data | autosharpened map from Phenix (at 3.5 A) based on RELION 3D autorefinement map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | capsid / design / virus mimic / VIRUS LIKE PARTICLE | |||||||||
| Function / homology |  Function and homology information Function and homology information6,7-dimethyl-8-ribityllumazine synthase / 6,7-dimethyl-8-ribityllumazine synthase activity / riboflavin synthase complex / riboflavin biosynthetic process / RNA polymerase binding / transcription antitermination factor activity, RNA binding / bacterial-type RNA polymerase core enzyme binding / regulation of DNA-templated transcription elongation / transcription antitermination / DNA-templated transcription termination ...6,7-dimethyl-8-ribityllumazine synthase / 6,7-dimethyl-8-ribityllumazine synthase activity / riboflavin synthase complex / riboflavin biosynthetic process / RNA polymerase binding / transcription antitermination factor activity, RNA binding / bacterial-type RNA polymerase core enzyme binding / regulation of DNA-templated transcription elongation / transcription antitermination / DNA-templated transcription termination / RNA stem-loop binding / single-stranded RNA binding / regulation of transcription by RNA polymerase II / DNA binding / RNA binding / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  Escherichia virus lambda / Escherichia virus lambda /   Aquifex aeolicus VF5 (bacteria) Aquifex aeolicus VF5 (bacteria) | |||||||||
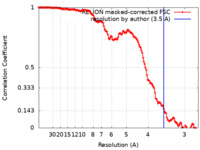
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Tetter S / Hilvert D | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2021 Journal: Science / Year: 2021Title: Evolution of a virus-like architecture and packaging mechanism in a repurposed bacterial protein. Authors: Stephan Tetter / Naohiro Terasaka / Angela Steinauer / Richard J Bingham / Sam Clark / Andrew J P Scott / Nikesh Patel / Marc Leibundgut / Emma Wroblewski / Nenad Ban / Peter G Stockley / ...Authors: Stephan Tetter / Naohiro Terasaka / Angela Steinauer / Richard J Bingham / Sam Clark / Andrew J P Scott / Nikesh Patel / Marc Leibundgut / Emma Wroblewski / Nenad Ban / Peter G Stockley / Reidun Twarock / Donald Hilvert /   Abstract: Viruses are ubiquitous pathogens of global impact. Prompted by the hypothesis that their earliest progenitors recruited host proteins for virion formation, we have used stringent laboratory evolution ...Viruses are ubiquitous pathogens of global impact. Prompted by the hypothesis that their earliest progenitors recruited host proteins for virion formation, we have used stringent laboratory evolution to convert a bacterial enzyme that lacks affinity for nucleic acids into an artificial nucleocapsid that efficiently packages and protects multiple copies of its own encoding messenger RNA. Revealing remarkable convergence on the molecular hallmarks of natural viruses, the accompanying changes reorganized the protein building blocks into an interlaced 240-subunit icosahedral capsid that is impermeable to nucleases, and emergence of a robust RNA stem-loop packaging cassette ensured high encapsidation yields and specificity. In addition to evincing a plausible evolutionary pathway for primordial viruses, these findings highlight practical strategies for developing nonviral carriers for diverse vaccine and delivery applications. #1:  Journal: Biorxiv / Year: 2021 Journal: Biorxiv / Year: 2021Title: Evolution of a virus-like architecture and packaging mechanism in a repurposed bacterial protein Authors: Tetter S / Terasaka N / Steinauer A / Bingham RJ / Clark S / Scott AJP / Patel N / Leibundgut M / Wroblewski E / Ban N / Stockley PG / Twarock R / Hilvert D | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_11631.map.gz emd_11631.map.gz | 223.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-11631-v30.xml emd-11631-v30.xml emd-11631.xml emd-11631.xml | 23.1 KB 23.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_11631_fsc.xml emd_11631_fsc.xml | 14.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_11631.png emd_11631.png | 91 KB | ||
| Masks |  emd_11631_msk_1.map emd_11631_msk_1.map | 244.1 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-11631.cif.gz emd-11631.cif.gz | 6.2 KB | ||
| Others |  emd_11631_additional_1.map.gz emd_11631_additional_1.map.gz emd_11631_additional_2.map.gz emd_11631_additional_2.map.gz emd_11631_half_map_1.map.gz emd_11631_half_map_1.map.gz emd_11631_half_map_2.map.gz emd_11631_half_map_2.map.gz | 229 MB 193.8 MB 194.1 MB 194.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-11631 http://ftp.pdbj.org/pub/emdb/structures/EMD-11631 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11631 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11631 | HTTPS FTP |
-Related structure data
| Related structure data |  7a4fMC  7a4gC  7a4hC  7a4iC  7a4jC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_11631.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_11631.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | autosharpened map from Phenix (at 3.5 A) based on RELION 3D autorefinement map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.375 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_11631_msk_1.map emd_11631_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
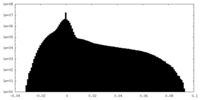
| Density Histograms |
-Additional map: postprocessed map from RELION
| File | emd_11631_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | postprocessed map from RELION | ||||||||||||
| Projections & Slices |
| ||||||||||||
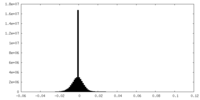
| Density Histograms |
-Additional map: 3D autorefinement in RELION
| File | emd_11631_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 3D autorefinement in RELION | ||||||||||||
| Projections & Slices |
| ||||||||||||
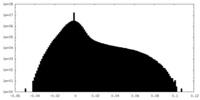
| Density Histograms |
-Half map: half map 1
| File | emd_11631_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
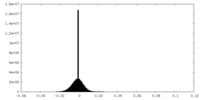
| Density Histograms |
-Half map: half map 2
| File | emd_11631_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Aquifex aeolicus lumazine synthase-derived nucleocapsid variant N...
| Entire | Name: Aquifex aeolicus lumazine synthase-derived nucleocapsid variant NC-1 (120-mer) |
|---|---|
| Components |
|
-Supramolecule #1: Aquifex aeolicus lumazine synthase-derived nucleocapsid variant N...
| Supramolecule | Name: Aquifex aeolicus lumazine synthase-derived nucleocapsid variant NC-1 (120-mer) type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Escherichia virus lambda Escherichia virus lambda |
-Macromolecule #1: Antitermination protein N,6,7-dimethyl-8-ribityllumazine synthase...
| Macromolecule | Name: Antitermination protein N,6,7-dimethyl-8-ribityllumazine synthase,6,7-dimethyl-8-ribityllumazine synthase type: protein_or_peptide / ID: 1 / Number of copies: 120 / Enantiomer: LEVO / EC number: 6,7-dimethyl-8-ribityllumazine synthase |
|---|---|
| Source (natural) | Organism:   Aquifex aeolicus VF5 (bacteria) Aquifex aeolicus VF5 (bacteria) |
| Molecular weight | Theoretical: 21.337312 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGNAKTRRRE RRAEKQAQWK AANAGAGAGA MATPHFDYIA SEVSKGLANL SLELRKPITF GVITADTLEQ AIERAGTKHG NKGWEAALS AIEMANLFKS LRGTGHHHHH HGSSMEIYEG KLTAEGLRFG IVASRFNHAL VDRLVEGAID CIVRHGGREE D ITLVRVPG ...String: MGNAKTRRRE RRAEKQAQWK AANAGAGAGA MATPHFDYIA SEVSKGLANL SLELRKPITF GVITADTLEQ AIERAGTKHG NKGWEAALS AIEMANLFKS LRGTGHHHHH HGSSMEIYEG KLTAEGLRFG IVASRFNHAL VDRLVEGAID CIVRHGGREE D ITLVRVPG SWEIPVAAGE LARKEDIDAV IAIGVLIRG UniProtKB: Antitermination protein N, 6,7-dimethyl-8-ribityllumazine synthase, 6,7-dimethyl-8-ribityllumazine synthase |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 200 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 15 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 22 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: INTEGRATING / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.6 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 130000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)