[English] 日本語
 Yorodumi
Yorodumi- EMDB-11591: Bacillus endospore appendages form a novel family of disulfide-li... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-11591 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Bacillus endospore appendages form a novel family of disulfide-linked pili | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Pilli / Endospore / Gram-Positive / Helical reconstruction / Disulphide bridge / UNKNOWN FUNCTION | |||||||||
| Function / homology | Endospore appendages core / Endospore appendages / DUF3992 domain-containing protein Function and homology information Function and homology information | |||||||||
| Biological species |  | |||||||||
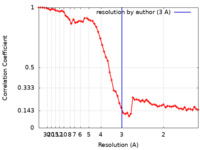
| Method | helical reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Pradhan B / Liedtke J | |||||||||
| Funding support |  Belgium, 1 items Belgium, 1 items
| |||||||||
 Citation Citation |  Journal: EMBO J / Year: 2021 Journal: EMBO J / Year: 2021Title: Endospore Appendages: a novel pilus superfamily from the endospores of pathogenic Bacilli. Authors: Brajabandhu Pradhan / Janine Liedtke / Mike Sleutel / Toril Lindbäck / Ephrem Debebe Zegeye / Kristin O Sullivan / Ann-Katrin Llarena / Ola Brynildsrud / Marina Aspholm / Han Remaut /   Abstract: Bacillus cereus sensu lato is a group of Gram-positive endospore-forming bacteria with high ecological diversity. Their endospores are decorated with micrometer-long appendages of unknown identity ...Bacillus cereus sensu lato is a group of Gram-positive endospore-forming bacteria with high ecological diversity. Their endospores are decorated with micrometer-long appendages of unknown identity and function. Here, we isolate endospore appendages (Enas) from the food poisoning outbreak strain B. cereus NVH 0075-95 and find proteinaceous fibers of two main morphologies: S- and L-Ena. By using cryoEM and 3D helical reconstruction of S-Enas, we show these to represent a novel class of Gram-positive pili. S-Enas consist of single domain subunits with jellyroll topology that are laterally stacked by β-sheet augmentation. S-Enas are longitudinally stabilized by disulfide bonding through N-terminal connector peptides that bridge the helical turns. Together, this results in flexible pili that are highly resistant to heat, drought, and chemical damage. Phylogenomic analysis reveals a ubiquitous presence of the ena-gene cluster in the B. cereus group, which include species of clinical, environmental, and food importance. We propose Enas to represent a new class of pili specifically adapted to the harsh conditions encountered by bacterial spores. #1:  Journal: Biorxiv / Year: 2020 Journal: Biorxiv / Year: 2020Title: Bacillus endospore appendages form a novel family of disulfide-linked pili Authors: Pradhan B / Liedtke J / Sleutel M / Lindback T / Llarena AK / Brynildsrud O / Aspholm M / Remaut H | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_11591.map.gz emd_11591.map.gz | 13.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-11591-v30.xml emd-11591-v30.xml emd-11591.xml emd-11591.xml | 13.1 KB 13.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_11591_fsc.xml emd_11591_fsc.xml | 7.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_11591.png emd_11591.png | 60.3 KB | ||
| Filedesc metadata |  emd-11591.cif.gz emd-11591.cif.gz | 5.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-11591 http://ftp.pdbj.org/pub/emdb/structures/EMD-11591 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11591 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11591 | HTTPS FTP |
-Related structure data
| Related structure data |  7a02MC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_11591.map.gz / Format: CCP4 / Size: 14.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_11591.map.gz / Format: CCP4 / Size: 14.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.784 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Ena1B
| Entire | Name: Ena1B |
|---|---|
| Components |
|
-Supramolecule #1: Ena1B
| Supramolecule | Name: Ena1B / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: all Details: In vitro assembled Bacillus endospore appendage comprising the subunit Ena1B |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: DUF3992 domain-containing protein
| Macromolecule | Name: DUF3992 domain-containing protein / type: protein_or_peptide / ID: 1 / Number of copies: 23 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 12.162675 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGNCSTNLSC CANGQKTIVQ DKVCIDWTAA ATAAIIYADN ISQDIYASGY LKVDTGTGPV TIVFYSGGVT GTAVETIVVA TGSSASFTV RRFDTVTILG TAAAETGEFC MTIRYTLS UniProtKB: DUF3992 domain-containing protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | helical array |
- Sample preparation
Sample preparation
| Buffer | pH: 7 / Component - Formula: H2O |
|---|---|
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Support film - Material: GRAPHENE OXIDE / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: OTHER |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 298.15 K / Instrument: GATAN CRYOPLUNGE 3 |
- Electron microscopy
Electron microscopy
| Microscope | JEOL CRYO ARM 300 |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 3000 / Average electron dose: 64.66 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated defocus max: 3.5 µm / Calibrated defocus min: 0.5 µm / Calibrated magnification: 60000 / Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: JEOL 3200FSC CRYOHOLDER |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL / Overall B value: 27.4 |
|---|---|
| Output model |  PDB-7a02: |
 Movie
Movie Controller
Controller









 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)