+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10428 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the two-fold capsomer of the dArc2 capsid | ||||||||||||
 Map data Map data | dArc2 two-fold capsomer | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | dArc / Gag / Virus / VLP / VIRUS LIKE PARTICLE | ||||||||||||
| Function / homology | Ty3 transposon capsid-like protein / Ty3 transposon capsid-like protein / virus-like capsid / extracellular vesicle / structural molecule activity / RNA binding / identical protein binding / membrane / Activity-regulated cytoskeleton associated protein 2 Function and homology information Function and homology information | ||||||||||||
| Biological species |  | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.7 Å | ||||||||||||
 Authors Authors | Erlendsson S / Morado DR | ||||||||||||
| Funding support |  Denmark, Denmark,  United States, United States,  United Kingdom, 3 items United Kingdom, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Neurosci / Year: 2020 Journal: Nat Neurosci / Year: 2020Title: Structures of virus-like capsids formed by the Drosophila neuronal Arc proteins. Authors: Simon Erlendsson / Dustin R Morado / Harrison B Cullen / Cedric Feschotte / Jason D Shepherd / John A G Briggs /   Abstract: Arc, a neuronal gene that is critical for synaptic plasticity, originated through the domestication of retrotransposon Gag genes and mediates intercellular messenger RNA transfer. We report high- ...Arc, a neuronal gene that is critical for synaptic plasticity, originated through the domestication of retrotransposon Gag genes and mediates intercellular messenger RNA transfer. We report high-resolution structures of retrovirus-like capsids formed by Drosophila dArc1 and dArc2 that have surface spikes and putative internal RNA-binding domains. These data demonstrate that virus-like capsid-forming properties of Arc are evolutionarily conserved and provide a structural basis for understanding their function in intercellular communication. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10428.map.gz emd_10428.map.gz | 8.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10428-v30.xml emd-10428-v30.xml emd-10428.xml emd-10428.xml | 16.9 KB 16.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_10428.png emd_10428.png | 87.6 KB | ||
| Filedesc metadata |  emd-10428.cif.gz emd-10428.cif.gz | 6.2 KB | ||
| Others |  emd_10428_additional.map.gz emd_10428_additional.map.gz | 9.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10428 http://ftp.pdbj.org/pub/emdb/structures/EMD-10428 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10428 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10428 | HTTPS FTP |
-Validation report
| Summary document |  emd_10428_validation.pdf.gz emd_10428_validation.pdf.gz | 502.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_10428_full_validation.pdf.gz emd_10428_full_validation.pdf.gz | 502.2 KB | Display | |
| Data in XML |  emd_10428_validation.xml.gz emd_10428_validation.xml.gz | 5.2 KB | Display | |
| Data in CIF |  emd_10428_validation.cif.gz emd_10428_validation.cif.gz | 5.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10428 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10428 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10428 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10428 | HTTPS FTP |
-Related structure data
| Related structure data |  6tauMC  6tapC  6taqC  6tarC  6tasC  6tatC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_10428.map.gz / Format: CCP4 / Size: 12.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10428.map.gz / Format: CCP4 / Size: 12.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | dArc2 two-fold capsomer | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.388 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
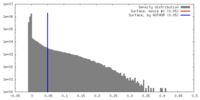
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: dArc2 two-fold capsomer unsharpened
| File | emd_10428_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | dArc2 two-fold capsomer unsharpened | ||||||||||||
| Projections & Slices |
| ||||||||||||
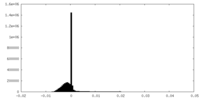
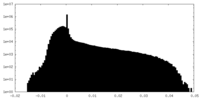
| Density Histograms |
- Sample components
Sample components
-Entire : dArc2 Capsids
| Entire | Name: dArc2 Capsids |
|---|---|
| Components |
|
-Supramolecule #1: dArc2 Capsids
| Supramolecule | Name: dArc2 Capsids / type: cell / ID: 1 / Parent: 0 / Macromolecule list: all Details: The two-fold dArc2 capsomer map is generated by symmetry expansion, sub-boxing and local refinement. |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Activity-regulated cytoskeleton associated protein 2
| Macromolecule | Name: Activity-regulated cytoskeleton associated protein 2 / type: protein_or_peptide / ID: 1 / Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 22.656734 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTQMSDEQFR IFIETIKSLG PIKEEPPSKG SFSNCTVRFS GQRDHDAVDE FINAVETYKE VEGISDKDAL KGLPLLFKSI AVVWWKGVR RDAKTWSDAL QLLRDHFSPT KPSYQIYMEI FETKQSYDEV IDSFICKQRA LLAKLPEGRH DEETELDFIY G LMQPKYRE ...String: MTQMSDEQFR IFIETIKSLG PIKEEPPSKG SFSNCTVRFS GQRDHDAVDE FINAVETYKE VEGISDKDAL KGLPLLFKSI AVVWWKGVR RDAKTWSDAL QLLRDHFSPT KPSYQIYMEI FETKQSYDEV IDSFICKQRA LLAKLPEGRH DEETELDFIY G LMQPKYRE SIPRHEVKTF RELLDRGRTV ERTRH UniProtKB: Activity-regulated cytoskeleton associated protein 2 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.1 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| |||||||||||||||
| Grid | Model: Homemade / Material: COPPER / Mesh: 300 / Support film - #0 - Film type ID: 1 / Support film - #0 - Material: CARBON / Support film - #0 - topology: LACEY / Support film - #1 - Film type ID: 2 / Support film - #1 - Material: CARBON / Support film - #1 - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 45 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.039 kPa / Details: 25 mA | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 278 K / Instrument: FEI VITROBOT MARK IV | |||||||||||||||
| Details | dArc2 capsids |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Digitization - Frames/image: 1-75 / Average electron dose: 35.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)