[English] 日本語
 Yorodumi
Yorodumi- EMDB-10384: A structure of the EIAV CA-SP hexamer (C6) from Gag-deltaMA spher... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10384 | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
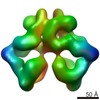
| Title | A structure of the EIAV CA-SP hexamer (C6) from Gag-deltaMA spheres assembled at pH6 | |||||||||||||||||||||||||||||||||
 Map data Map data | A 3.7Angstrom structure of the EIAV CA-SP hexamer (C6) from Gag-deltaMA spheres assembled at pH6 | |||||||||||||||||||||||||||||||||
 Sample Sample |
| |||||||||||||||||||||||||||||||||
 Keywords Keywords | Retrovirus / lentivirus / Equine infectious anemia virus / EIAV / Gag / capsid / IP6 / phytic acid / inositolhexakiphosphate / VIRAL PROTEIN | |||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationviral budding via host ESCRT complex / viral nucleocapsid / structural constituent of virion / nucleic acid binding / zinc ion binding Similarity search - Function | |||||||||||||||||||||||||||||||||
| Biological species |  Equine infectious anemia virus Equine infectious anemia virus | |||||||||||||||||||||||||||||||||
| Method | subtomogram averaging / cryo EM / Resolution: 3.7 Å | |||||||||||||||||||||||||||||||||
 Authors Authors | Dick RA / Xu C | |||||||||||||||||||||||||||||||||
| Funding support |  Austria, Austria,  United States, United States,  United Kingdom, United Kingdom,  Germany, 10 items Germany, 10 items
| |||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: PLoS Pathog / Year: 2020 Journal: PLoS Pathog / Year: 2020Title: Structures of immature EIAV Gag lattices reveal a conserved role for IP6 in lentivirus assembly. Authors: Robert A Dick / Chaoyi Xu / Dustin R Morado / Vladyslav Kravchuk / Clifton L Ricana / Terri D Lyddon / Arianna M Broad / J Ryan Feathers / Marc C Johnson / Volker M Vogt / Juan R Perilla / ...Authors: Robert A Dick / Chaoyi Xu / Dustin R Morado / Vladyslav Kravchuk / Clifton L Ricana / Terri D Lyddon / Arianna M Broad / J Ryan Feathers / Marc C Johnson / Volker M Vogt / Juan R Perilla / John A G Briggs / Florian K M Schur /     Abstract: Retrovirus assembly is driven by the multidomain structural protein Gag. Interactions between the capsid domains (CA) of Gag result in Gag multimerization, leading to an immature virus particle that ...Retrovirus assembly is driven by the multidomain structural protein Gag. Interactions between the capsid domains (CA) of Gag result in Gag multimerization, leading to an immature virus particle that is formed by a protein lattice based on dimeric, trimeric, and hexameric protein contacts. Among retroviruses the inter- and intra-hexamer contacts differ, especially in the N-terminal sub-domain of CA (CANTD). For HIV-1 the cellular molecule inositol hexakisphosphate (IP6) interacts with and stabilizes the immature hexamer, and is required for production of infectious virus particles. We have used in vitro assembly, cryo-electron tomography and subtomogram averaging, atomistic molecular dynamics simulations and mutational analyses to study the HIV-related lentivirus equine infectious anemia virus (EIAV). In particular, we sought to understand the structural conservation of the immature lentivirus lattice and the role of IP6 in EIAV assembly. Similar to HIV-1, IP6 strongly promoted in vitro assembly of EIAV Gag proteins into virus-like particles (VLPs), which took three morphologically highly distinct forms: narrow tubes, wide tubes, and spheres. Structural characterization of these VLPs to sub-4Å resolution unexpectedly showed that all three morphologies are based on an immature lattice with preserved key structural components, highlighting the structural versatility of CA to form immature assemblies. A direct comparison between EIAV and HIV revealed that both lentiviruses maintain similar immature interfaces, which are established by both conserved and non-conserved residues. In both EIAV and HIV-1, IP6 regulates immature assembly via conserved lysine residues within the CACTD and SP. Lastly, we demonstrate that IP6 stimulates in vitro assembly of immature particles of several other retroviruses in the lentivirus genus, suggesting a conserved role for IP6 in lentiviral assembly. | |||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10384.map.gz emd_10384.map.gz | 46.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10384-v30.xml emd-10384-v30.xml emd-10384.xml emd-10384.xml | 20.8 KB 20.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_10384.png emd_10384.png | 244.7 KB | ||
| Filedesc metadata |  emd-10384.cif.gz emd-10384.cif.gz | 7.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10384 http://ftp.pdbj.org/pub/emdb/structures/EMD-10384 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10384 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10384 | HTTPS FTP |
-Validation report
| Summary document |  emd_10384_validation.pdf.gz emd_10384_validation.pdf.gz | 619.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_10384_full_validation.pdf.gz emd_10384_full_validation.pdf.gz | 618.9 KB | Display | |
| Data in XML |  emd_10384_validation.xml.gz emd_10384_validation.xml.gz | 6.1 KB | Display | |
| Data in CIF |  emd_10384_validation.cif.gz emd_10384_validation.cif.gz | 7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10384 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10384 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10384 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10384 | HTTPS FTP |
-Related structure data
| Related structure data |  6t64MC  6t61C  6t63C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_10384.map.gz / Format: CCP4 / Size: 50.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10384.map.gz / Format: CCP4 / Size: 50.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | A 3.7Angstrom structure of the EIAV CA-SP hexamer (C6) from Gag-deltaMA spheres assembled at pH6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.041 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Equine infectious anemia virus
| Entire | Name:  Equine infectious anemia virus Equine infectious anemia virus |
|---|---|
| Components |
|
-Supramolecule #1: Equine infectious anemia virus
| Supramolecule | Name: Equine infectious anemia virus / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all Details: Gag construct was expressed in E.coli and purified using the SUMO-tag system. Assembly was performed at pH6. NCBI-ID: 11665 / Sci species name: Equine infectious anemia virus / Virus type: VIRUS-LIKE PARTICLE / Virus isolate: OTHER / Virus enveloped: No / Virus empty: Yes |
|---|---|
| Host (natural) | Organism:  |
| Virus shell | Shell ID: 1 / Name: Capsid / Diameter: 1000.0 Å |
-Macromolecule #1: Gag polyprotein
| Macromolecule | Name: Gag polyprotein / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Equine infectious anemia virus Equine infectious anemia virus |
| Molecular weight | Theoretical: 54.881535 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGDPLTWSKA LKKLEKVTVQ GSQKLTTGNC NWALSLVDLF HDTNFVKEKD WQLRDVIPLL EDVTQTLSGQ EREAFERTWW AISAVKMGL QINNVVDGKA SFQLLRAKYE KKTANKKQSE PSEEYPIMID GAGNRNFRPL TPRGYTTWVN TIQTNGLLNE A SQNLFGIL ...String: MGDPLTWSKA LKKLEKVTVQ GSQKLTTGNC NWALSLVDLF HDTNFVKEKD WQLRDVIPLL EDVTQTLSGQ EREAFERTWW AISAVKMGL QINNVVDGKA SFQLLRAKYE KKTANKKQSE PSEEYPIMID GAGNRNFRPL TPRGYTTWVN TIQTNGLLNE A SQNLFGIL SVDCTSEEMN AFLDVVPGQA GQKQILLDAI DKIADDWDNR HPLPNAPLVA PPQGPIPMTA RFIRGLGVPR ER QMEPAFD QFRQTYRQWI IEAMSEGIKV MIGKPKAQNI RQGAKEPYPE FVDRLLSQIK SEGHPQEISK FLTDTLTIQN ANE ECRNAM RHLRPEDTLE EKMYACRDIG TTKQKMMLLA KALQTGLAGP FKGGALKGGP LKAAQTCYNC GKPGHLSSQC RAPK VCFKC KQPGHFSKQC RSVPKNGKQG AQGRPQKQTF PIQQKSQHNK SVVQETPQTQ NLYPDLSEIK KEYNVKEKDQ VEDLN LDSL WE UniProtKB: Gag polyprotein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 6 Component:
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: C-flat-2/2 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: AIR / Details: 20 mA | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 15 K / Instrument: FEI VITROBOT MARK II / Details: 1-2 seconds blot time, offset -3mm. | ||||||||||||
| Details | Virus-like-particles (spherical) of EIAV Gag deltaMAdeltap9 (referred to as Gag deltaMA) assembled at pH6. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Quantum LS / Energy filter - Slit width: 20 eV |
| Details | nanoprobe |
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Dimensions - Width: 3708 pixel / Digitization - Dimensions - Height: 3838 pixel / Number grids imaged: 1 / Average exposure time: 1.4 sec. / Average electron dose: 3.4 e/Å2 Details: Data was acquired using a dose-symmetric tilt acquisition scheme, as described in Hagen et al, 2017, J. Struct. Biol, 197(2):191-8 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: -3.5 µm / Nominal defocus min: -1.5 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Details | Tilt series were low-pass filtered according to their cumulative dose using exposure filters that were calculated using an exposure-dependent amplitude attenuation function and critical exposure constants (as published in Grant & Grigorieff, Elife, 2015). Tilt series were aligned and reconstructed in IMOD. |
|---|---|
| Final reconstruction | Number classes used: 1 / Applied symmetry - Point group: C6 (6 fold cyclic) / Resolution.type: BY AUTHOR / Resolution: 3.7 Å / Resolution method: FSC 0.143 CUT-OFF / Software: (Name: AV3, TOM Toolbox) / Number subtomograms used: 64961 |
| Extraction | Number tomograms: 55 / Number images used: 311207 Method: Subvolumes were defined according to their position on the VLPs Software - Name:  MATLAB / Software - details: partially based on the TOM toolbox MATLAB / Software - details: partially based on the TOM toolboxDetails: Subtomogram extraction positions were defined in Amira using the electron microscopy toolbox by determing the radii and the center of the VLPs. Initially, positions were oversampled and ...Details: Subtomogram extraction positions were defined in Amira using the electron microscopy toolbox by determing the radii and the center of the VLPs. Initially, positions were oversampled and subsequently cleaned during alignments using cross-correlation and distance thresholds. |
| Final angle assignment | Type: PROJECTION MATCHING Projection matching processing - Number reference projections: 1 Projection matching processing - Merit function: CC / Software: (Name: AV3, TOM Toolbox) Details: Subtomogram alignment was performed as described in the published manuscript. |
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Details | Rigid body fitting was done in Chimera. Missing residues were built de novo in Coot. Refinement was performed iteratively in Phenix and Coot. |
| Refinement | Space: REAL / Protocol: OTHER / Target criteria: Cross-correlation coefficient |
| Output model |  PDB-6t64: |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






















