[English] 日本語
 Yorodumi
Yorodumi- EMDB-10353: Release factor-dependent ribosome rescue by BrfA in the Gram-posi... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10353 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Release factor-dependent ribosome rescue by BrfA in the Gram-positive bacterium Bacillus subtilis | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Cryo-EM / bacterial ribosome rescue / Bacillus ribosome rescue factor A / Bacillus subtilis peptide chain release factor 2 / RIBOSOME | |||||||||
| Function / homology |  Function and homology information Function and homology informationtranslation release factor activity, codon specific / negative regulation of cytoplasmic translational initiation / transcription antitermination factor activity, RNA binding / ornithine decarboxylase inhibitor activity / misfolded RNA binding / Group I intron splicing / RNA folding / transcriptional attenuation / endoribonuclease inhibitor activity / positive regulation of ribosome biogenesis ...translation release factor activity, codon specific / negative regulation of cytoplasmic translational initiation / transcription antitermination factor activity, RNA binding / ornithine decarboxylase inhibitor activity / misfolded RNA binding / Group I intron splicing / RNA folding / transcriptional attenuation / endoribonuclease inhibitor activity / positive regulation of ribosome biogenesis / RNA-binding transcription regulator activity / negative regulation of cytoplasmic translation / four-way junction DNA binding / DnaA-L2 complex / translation repressor activity / negative regulation of translational initiation / regulation of mRNA stability / negative regulation of DNA-templated DNA replication initiation / mRNA regulatory element binding translation repressor activity / positive regulation of RNA splicing / assembly of large subunit precursor of preribosome / cytosolic ribosome assembly / response to reactive oxygen species / regulation of DNA-templated transcription elongation / ribosome assembly / transcription elongation factor complex / transcription antitermination / DNA endonuclease activity / regulation of cell growth / translational initiation / DNA-templated transcription termination / response to radiation / maintenance of translational fidelity / mRNA 5'-UTR binding / regulation of translation / large ribosomal subunit / ribosome biogenesis / transferase activity / ribosome binding / ribosomal small subunit biogenesis / ribosomal small subunit assembly / ribosomal large subunit assembly / 5S rRNA binding / small ribosomal subunit / small ribosomal subunit rRNA binding / cytosolic small ribosomal subunit / large ribosomal subunit rRNA binding / cytosolic large ribosomal subunit / cytoplasmic translation / tRNA binding / negative regulation of translation / rRNA binding / structural constituent of ribosome / ribosome / translation / hydrolase activity / viral translational frameshifting / response to antibiotic / negative regulation of DNA-templated transcription / mRNA binding / DNA binding / RNA binding / zinc ion binding / membrane / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |    | |||||||||
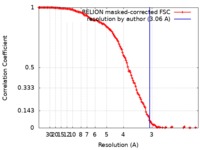
| Method | single particle reconstruction / cryo EM / Resolution: 3.06 Å | |||||||||
 Authors Authors | Muller C / Beckert B | |||||||||
| Funding support |  Germany, 1 items Germany, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2019 Journal: Nat Commun / Year: 2019Title: Release factor-dependent ribosome rescue by BrfA in the Gram-positive bacterium Bacillus subtilis. Authors: Naomi Shimokawa-Chiba / Claudia Müller / Keigo Fujiwara / Bertrand Beckert / Koreaki Ito / Daniel N Wilson / Shinobu Chiba /   Abstract: Rescue of the ribosomes from dead-end translation complexes, such as those on truncated (non-stop) mRNA, is essential for the cell. Whereas bacteria use trans-translation for ribosome rescue, some ...Rescue of the ribosomes from dead-end translation complexes, such as those on truncated (non-stop) mRNA, is essential for the cell. Whereas bacteria use trans-translation for ribosome rescue, some Gram-negative species possess alternative and release factor (RF)-dependent rescue factors, which enable an RF to catalyze stop-codon-independent polypeptide release. We now discover that the Gram-positive Bacillus subtilis has an evolutionarily distinct ribosome rescue factor named BrfA. Genetic analysis shows that B. subtilis requires the function of either trans-translation or BrfA for growth, even in the absence of proteotoxic stresses. Biochemical and cryo-electron microscopy (cryo-EM) characterization demonstrates that BrfA binds to non-stop stalled ribosomes, recruits homologous RF2, but not RF1, and induces its transition into an open active conformation. Although BrfA is distinct from E. coli ArfA, they use convergent strategies in terms of mode of action and expression regulation, indicating that many bacteria may have evolved as yet unidentified ribosome rescue systems. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10353.map.gz emd_10353.map.gz | 20 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10353-v30.xml emd-10353-v30.xml emd-10353.xml emd-10353.xml | 85.5 KB 85.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_10353_fsc.xml emd_10353_fsc.xml | 13.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_10353.png emd_10353.png | 40.2 KB | ||
| Filedesc metadata |  emd-10353.cif.gz emd-10353.cif.gz | 14.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10353 http://ftp.pdbj.org/pub/emdb/structures/EMD-10353 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10353 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10353 | HTTPS FTP |
-Related structure data
| Related structure data |  6szsMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_10353.map.gz / Format: CCP4 / Size: 190.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10353.map.gz / Format: CCP4 / Size: 190.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.065 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : E. coli 70S stalled on truncated mRNA in complex BsBrfA and BsRF2...
+Supramolecule #1: E. coli 70S stalled on truncated mRNA in complex BsBrfA and BsRF2...
+Supramolecule #2: Escherichia coli ribosome with P-site tRNA-Pro(CGG, carrying the ...
+Supramolecule #3: Bacillus subtilis ribosome rescue factor (BrfA; former YqkK) and ...
+Macromolecule #1: 50S ribosomal protein L32
+Macromolecule #2: 50S ribosomal protein L33
+Macromolecule #3: 50S ribosomal protein L34
+Macromolecule #4: 50S ribosomal protein L35
+Macromolecule #5: 50S ribosomal protein L36
+Macromolecule #6: 50S ribosomal protein L31
+Macromolecule #9: 50S ribosomal protein L2
+Macromolecule #10: 50S ribosomal protein L3
+Macromolecule #11: 50S ribosomal protein L4
+Macromolecule #12: 50S ribosomal protein L5
+Macromolecule #13: 50S ribosomal protein L6
+Macromolecule #14: 50S ribosomal protein L9
+Macromolecule #15: 50S ribosomal protein L13
+Macromolecule #16: 50S ribosomal protein L14
+Macromolecule #17: 50S ribosomal protein L15
+Macromolecule #18: 50S ribosomal protein L16
+Macromolecule #19: 50S ribosomal protein L17
+Macromolecule #20: 50S ribosomal protein L18
+Macromolecule #21: 50S ribosomal protein L19
+Macromolecule #22: 50S ribosomal protein L20
+Macromolecule #23: 50S ribosomal protein L21
+Macromolecule #24: 50S ribosomal protein L22
+Macromolecule #25: 50S ribosomal protein L23
+Macromolecule #26: 50S ribosomal protein L24
+Macromolecule #27: 50S ribosomal protein L25
+Macromolecule #28: 50S ribosomal protein L27
+Macromolecule #29: 50S ribosomal protein L28
+Macromolecule #30: 50S ribosomal protein L29
+Macromolecule #31: 50S ribosomal protein L30
+Macromolecule #33: 30S ribosomal protein S2
+Macromolecule #34: 30S ribosomal protein S3
+Macromolecule #35: 30S ribosomal protein S4
+Macromolecule #36: 30S ribosomal protein S5
+Macromolecule #37: 30S ribosomal protein S6
+Macromolecule #38: 30S ribosomal protein S7
+Macromolecule #39: 30S ribosomal protein S8
+Macromolecule #40: 30S ribosomal protein S9
+Macromolecule #41: 30S ribosomal protein S10
+Macromolecule #42: 30S ribosomal protein S11
+Macromolecule #43: 30S ribosomal protein S12
+Macromolecule #44: 30S ribosomal protein S13
+Macromolecule #45: 30S ribosomal protein S14
+Macromolecule #46: 30S ribosomal protein S15
+Macromolecule #47: 30S ribosomal protein S16
+Macromolecule #48: 30S ribosomal protein S17
+Macromolecule #49: 30S ribosomal protein S18
+Macromolecule #50: 30S ribosomal protein S19
+Macromolecule #51: 30S ribosomal protein S20
+Macromolecule #52: 30S ribosomal protein S21
+Macromolecule #55: Uncharacterized protein YqkK
+Macromolecule #56: Peptide chain release factor 2
+Macromolecule #7: 23S ribosomal RNA
+Macromolecule #8: 5S ribosomal RNA
+Macromolecule #32: 16S ribosomal RNA
+Macromolecule #53: mRNA
+Macromolecule #54: P-site tRNA-Pro(CGG)
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.2 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Average electron dose: 83.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)