[English] 日本語
 Yorodumi
Yorodumi- EMDB-10064: Structure of s-Mgm1 decorating the inner surface of tubulated lip... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10064 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
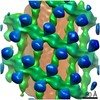
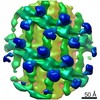
| Title | Structure of s-Mgm1 decorating the inner surface of tubulated lipid membranes | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | mitochondrial protein / membrane remodelling / GTPase / mitochondrial dynamics / subtomogram averaging / contractile protein | |||||||||
| Function / homology |  Function and homology information Function and homology informationmembrane bending activity / GTPase-dependent fusogenic activity / dynamin GTPase / receptor internalization / mitochondrial intermembrane space / microtubule binding / microtubule / mitochondrial inner membrane / GTPase activity / lipid binding ...membrane bending activity / GTPase-dependent fusogenic activity / dynamin GTPase / receptor internalization / mitochondrial intermembrane space / microtubule binding / microtubule / mitochondrial inner membrane / GTPase activity / lipid binding / GTP binding / metal ion binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Chaetomium thermophilum var. thermophilum DSM 1495 (fungus) Chaetomium thermophilum var. thermophilum DSM 1495 (fungus) | |||||||||
| Method | subtomogram averaging / cryo EM / Resolution: 20.6 Å | |||||||||
 Authors Authors | Faelber K / Dietrich L / Noel JK / Sanchez R / Kudryashev M / Kuelbrandt W / Daumke O / Chiaruttin N / Lilie H / Schleger J ...Faelber K / Dietrich L / Noel JK / Sanchez R / Kudryashev M / Kuelbrandt W / Daumke O / Chiaruttin N / Lilie H / Schleger J / Rosenbaum E / Hessenberger M / Matthaeus C / Noe F / Roux A / van der Laan M / Kuehlbrandt W | |||||||||
| Funding support |  Germany, 1 items Germany, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2019 Journal: Nature / Year: 2019Title: Structure and assembly of the mitochondrial membrane remodelling GTPase Mgm1. Authors: Katja Faelber / Lea Dietrich / Jeffrey K Noel / Florian Wollweber / Anna-Katharina Pfitzner / Alexander Mühleip / Ricardo Sánchez / Misha Kudryashev / Nicolas Chiaruttini / Hauke Lilie / ...Authors: Katja Faelber / Lea Dietrich / Jeffrey K Noel / Florian Wollweber / Anna-Katharina Pfitzner / Alexander Mühleip / Ricardo Sánchez / Misha Kudryashev / Nicolas Chiaruttini / Hauke Lilie / Jeanette Schlegel / Eva Rosenbaum / Manuel Hessenberger / Claudia Matthaeus / Séverine Kunz / Alexander von der Malsburg / Frank Noé / Aurélien Roux / Martin van der Laan / Werner Kühlbrandt / Oliver Daumke /   Abstract: Balanced fusion and fission are key for the proper function and physiology of mitochondria. Remodelling of the mitochondrial inner membrane is mediated by the dynamin-like protein mitochondrial ...Balanced fusion and fission are key for the proper function and physiology of mitochondria. Remodelling of the mitochondrial inner membrane is mediated by the dynamin-like protein mitochondrial genome maintenance 1 (Mgm1) in fungi or the related protein optic atrophy 1 (OPA1) in animals. Mgm1 is required for the preservation of mitochondrial DNA in yeast, whereas mutations in the OPA1 gene in humans are a common cause of autosomal dominant optic atrophy-a genetic disorder that affects the optic nerve. Mgm1 and OPA1 are present in mitochondria as a membrane-integral long form and a short form that is soluble in the intermembrane space. Yeast strains that express temperature-sensitive mutants of Mgm1 or mammalian cells that lack OPA1 display fragmented mitochondria, which suggests that Mgm1 and OPA1 have an important role in inner-membrane fusion. Consistently, only the mitochondrial outer membrane-not the inner membrane-fuses in the absence of functional Mgm1. Mgm1 and OPA1 have also been shown to maintain proper cristae architecture; for example, OPA1 prevents the release of pro-apoptotic factors by tightening crista junctions. Finally, the short form of OPA1 localizes to mitochondrial constriction sites, where it presumably promotes mitochondrial fission. How Mgm1 and OPA1 perform their diverse functions in membrane fusion, scission and cristae organization is at present unknown. Here we present crystal and electron cryo-tomography structures of Mgm1 from Chaetomium thermophilum. Mgm1 consists of a GTPase (G) domain, a bundle signalling element domain, a stalk, and a paddle domain that contains a membrane-binding site. Biochemical and cell-based experiments demonstrate that the Mgm1 stalk mediates the assembly of bent tetramers into helical filaments. Electron cryo-tomography studies of Mgm1-decorated lipid tubes and fluorescence microscopy experiments on reconstituted membrane tubes indicate how the tetramers assemble on positively or negatively curved membranes. Our findings convey how Mgm1 and OPA1 filaments dynamically remodel the mitochondrial inner membrane. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10064.map.gz emd_10064.map.gz | 4.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10064-v30.xml emd-10064-v30.xml emd-10064.xml emd-10064.xml | 19.2 KB 19.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_10064_fsc.xml emd_10064_fsc.xml | 5.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_10064.png emd_10064.png | 161.5 KB | ||
| Masks |  emd_10064_msk_1.map emd_10064_msk_1.map | 15.6 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-10064.cif.gz emd-10064.cif.gz | 6.6 KB | ||
| Others |  emd_10064_half_map_1.map.gz emd_10064_half_map_1.map.gz emd_10064_half_map_2.map.gz emd_10064_half_map_2.map.gz | 14.5 MB 14.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10064 http://ftp.pdbj.org/pub/emdb/structures/EMD-10064 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10064 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10064 | HTTPS FTP |
-Related structure data
| Related structure data |  6rzvMC  4584C  6ql4C  6rztC  6rzuC  6rzwC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_10064.map.gz / Format: CCP4 / Size: 15.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10064.map.gz / Format: CCP4 / Size: 15.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.7 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_10064_msk_1.map emd_10064_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Unfiltered non-masked half-map #1
| File | emd_10064_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unfiltered non-masked half-map #1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Unfiltered non-masked half-map #2
| File | emd_10064_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unfiltered non-masked half-map #2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Mgm1
| Entire | Name: Mgm1 |
|---|---|
| Components |
|
-Supramolecule #1: Mgm1
| Supramolecule | Name: Mgm1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: short isoform with C- and N-terminal truncations |
|---|---|
| Source (natural) | Organism:  Chaetomium thermophilum var. thermophilum DSM 1495 (fungus) Chaetomium thermophilum var. thermophilum DSM 1495 (fungus) |
-Macromolecule #1: Putative mitochondrial dynamin protein
| Macromolecule | Name: Putative mitochondrial dynamin protein / type: protein_or_peptide / ID: 1 / Number of copies: 16 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Chaetomium thermophilum var. thermophilum DSM 1495 (fungus) Chaetomium thermophilum var. thermophilum DSM 1495 (fungus) |
| Molecular weight | Theoretical: 77.360172 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: EEIMRDDNMM FITKKMIEIR NLLQKVGQGS TVTLPSIVVI GSQSSGKSSV LEAIVGHEFL PKGSNMITRR PIELTLVNDP EAKVDYGEF PDLGLARVTD FSLIQKTLTE LNQSVPESEC VTDDPIRLTI HSPNIPDLSL IDLPGYIQVA GENQPRELKR K ITELCDKY ...String: EEIMRDDNMM FITKKMIEIR NLLQKVGQGS TVTLPSIVVI GSQSSGKSSV LEAIVGHEFL PKGSNMITRR PIELTLVNDP EAKVDYGEF PDLGLARVTD FSLIQKTLTE LNQSVPESEC VTDDPIRLTI HSPNIPDLSL IDLPGYIQVA GENQPRELKR K ITELCDKY IRGPNIILAI SAADTDLANS TALQASRRVD PRGERTIGVI TKMDLVEPEK GAAILSDRQY PLKLGYVGVI SK LPPQSGL FRRDTGNLLA SINRNEKNYF GSHPTEFGPD SGVSTGVMTL RKKLLQVLEQ QMSSKLNETT EAIQRELEET TYQ FKVQYN EQPMSAESYL AASLDDFKHQ FHEFASSFGR PQLQTLLKDA LDQKVLDQLA ARYWNRPIED LSPAPREPDN IIDL PKADP DSPYWHRQLD TACSGLTRLG VGRLAATVAA SAIQQHVEKL LDKSSFAKHP SARKVISDAA ATVLADRSYA TSDGI EISL KPYKFDPDIQ PNEWAQGREH VVGVLQAELE QCQAAMKALE NSVGGRKKLK EVMSFVDKAR KGEIIVEGDH PSGAGG FSA ALLARGREAV FLRDRADILS LRIQAAKSRQ CKTLTNKYYC PEVFLDAVAT KLAQTAVLFL NVEMLNDFYV RFPREVE AK LHEHMHAGGG LEKFAREDPK VRRHLDLIRR KELLETVLGK IEELHRISSG TAG UniProtKB: Dynamin-like GTPase MGM1, mitochondrial |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5 mg/mL |
|---|---|
| Buffer | pH: 7.4 / Details: 20 mM HEPES, 200 mM NaCl, residual MgCl2, 9mM KCl. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 70 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV |
| Details | The sample was prepared in the described buffer. Just before freezing 6 nm colloidal gold fiducial marker were added in a 1:1 ratio |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 2.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 2.0 µm / Nominal magnification: 53000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)