[English] 日本語
 Yorodumi
Yorodumi- EMDB-30158: Epstein-Barr virus, C1 portal-proximal penton vertex, CATC binding -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-30158 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Epstein-Barr virus, C1 portal-proximal penton vertex, CATC binding | ||||||||||||||||||
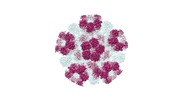
 Map data Map data | For the entry D_1300016325, the EM map (C1) authors have provided shows a pseudo-C5 symmetry with four "ASU" copies are identity to that provided in other related entry D_1300016276. The coordinates authors uploaded only represents the unique one "ASU" due to the limitation of the chain numbers (beyond 62 chains if the model could overlap with the whole map). | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | portal-proximal penton vertex / CATC binding / tegumented capsid / asymmetric reconstruction / VIRAL PROTEIN | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationT=16 icosahedral viral capsid / viral genome packaging / viral tegument / viral capsid assembly / symbiont-mediated suppression of host TRAF-mediated signal transduction / viral process / chromosome organization / viral penetration into host nucleus / viral capsid / host cell ...T=16 icosahedral viral capsid / viral genome packaging / viral tegument / viral capsid assembly / symbiont-mediated suppression of host TRAF-mediated signal transduction / viral process / chromosome organization / viral penetration into host nucleus / viral capsid / host cell / symbiont-mediated perturbation of host ubiquitin-like protein modification / host cell cytoplasm / ubiquitinyl hydrolase 1 / cysteine-type deubiquitinase activity / Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases / symbiont entry into host cell / host cell nucleus / structural molecule activity / proteolysis / DNA binding Similarity search - Function | ||||||||||||||||||
| Biological species |  Epstein-Barr virus (strain B95-8) (Epstein-Barr virus (strain B95-8)) / Epstein-Barr virus (strain B95-8) (Epstein-Barr virus (strain B95-8)) /  Human gammaherpesvirus 4 (Epstein-Barr virus) Human gammaherpesvirus 4 (Epstein-Barr virus) | ||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.3 Å | ||||||||||||||||||
 Authors Authors | Li Z / Yu X / Zeng M | ||||||||||||||||||
| Funding support |  China, 5 items China, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Cell Res / Year: 2020 Journal: Cell Res / Year: 2020Title: CryoEM structure of the tegumented capsid of Epstein-Barr virus. Authors: Zhihai Li / Xiao Zhang / Lili Dong / Jingjing Pang / Miao Xu / Qian Zhong / Mu-Sheng Zeng / Xuekui Yu /  Abstract: Epstein-Barr virus (EBV) is the primary cause of infectious mononucleosis and has been shown to be closely associated with various malignancies. Here, we present a complete atomic model of EBV, ...Epstein-Barr virus (EBV) is the primary cause of infectious mononucleosis and has been shown to be closely associated with various malignancies. Here, we present a complete atomic model of EBV, including the icosahedral capsid, the dodecameric portal and the capsid-associated tegument complex (CATC). Our in situ portal from the tegumented capsid adopts a closed conformation with its channel valve holding the terminal viral DNA and with its crown region firmly engaged by three layers of ring-like dsDNA, which, together with the penton flexibility, effectively alleviates the capsid inner pressure placed on the portal cap. In contrast, the CATCs, through binding to the flexible penton vertices in a stoichiometric manner, accurately increase the inner capsid pressure to facilitate the pressure-driven genome delivery. Together, our results provide important insights into the mechanism by which the EBV capsid, portal, packaged genome and the CATCs coordinately achieve a pressure balance to simultaneously benefit both viral genome retention and ejection. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_30158.map.gz emd_30158.map.gz | 93.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-30158-v30.xml emd-30158-v30.xml emd-30158.xml emd-30158.xml | 25.1 KB 25.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_30158.png emd_30158.png | 168.4 KB | ||
| Filedesc metadata |  emd-30158.cif.gz emd-30158.cif.gz | 10.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-30158 http://ftp.pdbj.org/pub/emdb/structures/EMD-30158 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30158 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30158 | HTTPS FTP |
-Related structure data
| Related structure data |  7br7MC  7bqtC  7bqxC  7br8C  7bsiC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_30158.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_30158.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | For the entry D_1300016325, the EM map (C1) authors have provided shows a pseudo-C5 symmetry with four "ASU" copies are identity to that provided in other related entry D_1300016276. The coordinates authors uploaded only represents the unique one "ASU" due to the limitation of the chain numbers (beyond 62 chains if the model could overlap with the whole map). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.31 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Human gammaherpesvirus 4
| Entire | Name:  Human gammaherpesvirus 4 (Epstein-Barr virus) Human gammaherpesvirus 4 (Epstein-Barr virus) |
|---|---|
| Components |
|
-Supramolecule #1: Human gammaherpesvirus 4
| Supramolecule | Name: Human gammaherpesvirus 4 / type: virus / ID: 1 / Parent: 0 / NCBI-ID: 10376 / Sci species name: Human gammaherpesvirus 4 / Virus type: VIRION / Virus isolate: STRAIN / Virus enveloped: Yes / Virus empty: No |
|---|
-Macromolecule #1: Capsid vertex component 2
| Macromolecule | Name: Capsid vertex component 2 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Epstein-Barr virus (strain B95-8) (Epstein-Barr virus (strain B95-8)) Epstein-Barr virus (strain B95-8) (Epstein-Barr virus (strain B95-8))Strain: B95-8 |
| Molecular weight | Theoretical: 62.525469 KDa |
| Sequence | String: MALSGHVLID PARLPRDTGP ELMWAPSLRN SLRVSPEALE LAEREAERAR SERWDRCAQV LKNRLLRVEL DGIMRDHLAR AEEIRQDLD AVVAFSDGLE SMQVRSPSTG GRSAPAPPSP SPAQPFTRLT GNAQYAVSIS PTDPPLMVAG SLAQTLLGNL Y GNINQWVP ...String: MALSGHVLID PARLPRDTGP ELMWAPSLRN SLRVSPEALE LAEREAERAR SERWDRCAQV LKNRLLRVEL DGIMRDHLAR AEEIRQDLD AVVAFSDGLE SMQVRSPSTG GRSAPAPPSP SPAQPFTRLT GNAQYAVSIS PTDPPLMVAG SLAQTLLGNL Y GNINQWVP SFGPWYRTMS ANAMQRRVFP KQLRGNLNFT NSVSLKLMTE VVAVLEGTTQ DFFSDVRHLP DLQAALILSV AY LLLQGGS SHQQRPLPAS REELLELGPE SLEKIIADLK AKSPGGNFMI LTSGNKEARQ SIAPLNRQAA YPPGTFADNK IYN LFVGAG LLPTTAALNV PGAAGRDRDL VYRIANQIFG EDVPPFSSHQ WNLRVGLAAL EALMLVYTLC ETANLAEAAT RRLH LSSLL PQAMQRRKPA MASAGMPGAY PVQTLFRHGE LFRFIWAHYV RPTVAADPQA SISSLFPGLV LLALELKLMD GQAPS HYAI NLTGQKFDTL FEIINQKLLF HDPAAMLAAR TQLRLAFEDG VGVALGRPSP MLAAREILER QFSASDDYDR LYFLTL GYL ASPVAPS UniProtKB: Capsid vertex component 2 |
-Macromolecule #2: Capsid vertex component 1
| Macromolecule | Name: Capsid vertex component 1 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Epstein-Barr virus (strain B95-8) (Epstein-Barr virus (strain B95-8)) Epstein-Barr virus (strain B95-8) (Epstein-Barr virus (strain B95-8))Strain: B95-8 |
| Molecular weight | Theoretical: 54.527941 KDa |
| Sequence | String: MDVHIDNQVL SGLGTPLLVH LFVPDTVMAE LCPNRVPNCE GAWCQTLFSD RTGLTRVCRV FAARGMLPGR PSHRGTFTSV PVYCDEGLP ELYNPFHVAA LRFYDEGGLV GELQIYYLSL FEGAKRALTD GHLIREASGV QESAAAMQPI PIDPGPPGGA G IEHMPVAA ...String: MDVHIDNQVL SGLGTPLLVH LFVPDTVMAE LCPNRVPNCE GAWCQTLFSD RTGLTRVCRV FAARGMLPGR PSHRGTFTSV PVYCDEGLP ELYNPFHVAA LRFYDEGGLV GELQIYYLSL FEGAKRALTD GHLIREASGV QESAAAMQPI PIDPGPPGGA G IEHMPVAA AQVEHPKTYD LKQILLEITQ EENRGEQRLG HAGSPALCLG LRLRAGAETK AAAETSVSKH HPALENPSNI RG SAGGEGG GGRAGTGGTV GVGSGALSRV PVSFSKTRRA IRESRALVRG IAHIFSPHAL YVVTYPELSA QGRLHRMTAV THA SPATDL AEVSILGAPE REFRFLISVA LRISASFREK LAMQAWTAQQ EIPVVIPTSY SRIYKNSDLI REAFFTVQTR VSWE SCWVK ATISNAPKTP DACLWIDSHP LYEEGASAWG KVIDSRPPGG LVGAASQLVA LGTDGHCVHL ATTSDGQAFL VLPGG FVIK GQLALTPEER GYILARHGIR REQ UniProtKB: Capsid vertex component 1 |
-Macromolecule #3: Large tegument protein deneddylase
| Macromolecule | Name: Large tegument protein deneddylase / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO / EC number: ubiquitinyl hydrolase 1 |
|---|---|
| Source (natural) | Organism:  Epstein-Barr virus (strain B95-8) (Epstein-Barr virus (strain B95-8)) Epstein-Barr virus (strain B95-8) (Epstein-Barr virus (strain B95-8))Strain: B95-8 |
| Molecular weight | Theoretical: 338.310625 KDa |
| Sequence | String: MSNGDWGQSQ RTRGTGPVRG IRTMDVNAPG GGSGGSALRI LGTASCNQAH CKFGRFAGIQ CVSNCVLYLV KSFLAGRPLT SRPELDEVL DEGARLDALM RQSGILKGHE MAQLTDVPSS VVLRGGGRVH IYRSAEIFGL VLFPAQIANS AVVQSLAEVL H GSYNGVAQ ...String: MSNGDWGQSQ RTRGTGPVRG IRTMDVNAPG GGSGGSALRI LGTASCNQAH CKFGRFAGIQ CVSNCVLYLV KSFLAGRPLT SRPELDEVL DEGARLDALM RQSGILKGHE MAQLTDVPSS VVLRGGGRVH IYRSAEIFGL VLFPAQIANS AVVQSLAEVL H GSYNGVAQ FILYICDIYA GAIIIETDGS FYLFDPHCQK DAAPGTPAHV RVSTYAHDIL QYVGAPGAQY TCVHLYFLPE AF ETEDPRI FMLEHYGVYD FYEANGSGFD LVGPELVSSD GEAAGTPGAD SSPPVMLPFE RRIIPYNLRP LPSRSFTSDS FPA ARYSPA KTNSPPSSPA SAAPASAAPA SAAPASAAPA SAAPASAAPA SAAPASAAPA SSPPLFIPIP GLGHTPGVPA PSTP PRASS GAAPQTPKRK KGLGKDSPHK KPTSGRRLPL SSTTDTEDDQ LPRTHVPPHR PPSAARLPPP VIPIPHQSPP ASPTP HPAP VSTIAPSVTP SPRLPLQIPI PLPQAAPSNP KIPLTTPSPS PTAAAAPTTT TLSPPPTQQQ PPQSAAPAPS PLLPQQ QPT PSAAPAPSPL LPQQQPPPSA ARAPSPLPPQ QQPLPSATPA PPPAQQLPPS ATTLEPEKNH PPAADRAGTE ISPSPPF GQ QPSFGDDASG GSGLVRYLSD LEEPFLSMSD SEEAESDLAS DIPTTEDEDM FEDEVFSNSL ESGSSAPTSP ITLDTARS Q YYQTTFDIET PEMDFVPLES NIARIAGHTY QEQAIVYDPA SNREVPEADA LSMIDYLLVT VVLEQGLIRS RDRSSVLNL LEFLKDWSGH LQVPTLDLEQ LLTSELNIQN LANMLSENKG RAGEFHKHLA AKLEACLPSL ATKDAVRVDA GAKMLAEIPQ LAESDDGKF DLEAARRRLT DLLSGGDQEA GEGGGEPEDN SIYRGPHVDV PLVLDDESWK RLLSLAEAAR TAVARQQAGV D EEDVRFLA LLTAIEYGAP PAASVPPFVH NVAVRSKNAA LHVRRCTADI RDKVASAASD YLSYLEDPSL PTVMDFDDLL TH LRHTCQI IASLPLLNIR YTSIEWDYRE LLYLGTALSD MSGIPWPLER VEEDDPSIAP LPEFETVAKK QKELETTREN EKR LRTILD DIEAMLGLAG VASAPGAPIS PASPSATPAN HDNPEATPPL ADTAALTIPV IEKYIANAGS IVGAAKNPTY IRLR DTIQQ IVRSKKYLMN ILKSITFYTI DNYIASFEES IDHLYRDLPV LDPEVQDGID RILDPMVSEA LHTFEMGNRL TLEPA RLVA LQNFATHSTL KETAAAVNLL PGLLAVYDAT ITGQAPEDAL RLLSGLQNQL SQTLIPGKLK KRFLSYLQKL KNNNND QLR QKEVQAWRLE AEGFKPATEE QLEAFLDTAP NKELKRQYEK KLRQLMETGR KEKEKLREQE DKERQERRAR EANEAWA RI RKALGARPEP APTSPDDWNT LLASLLPDNT DSAAAAAAAV ARNTDILDSL TQILAAMLLG ITRVRRERLR SLLVDDGG A AERMEAAEPG WFTDIETGPL ARLDAWPATP AATAKEGGGG RGAEEAAGAL FRARTAADAI RSALAQTRQA LQSPDMKSA VVNTDLEAPY AEYERGLAGL LEKRRAAEAA LTAIVSEYVD RTLPEATNDP GQANLPPPPT IPQATAPPRL ASDSALWPKK PQLLTRRER DDLLQATGDF FSELLTEAEA AEVRALEEQV RESQTLMAKA HEMAASTRRG FHTALEAVLS RSRDEAPDDE L RSLLPSPP KAPVQAPLEA ALARAAAGNG SWPYRKSLAA AKWIRGICEA VRGLSEGALA LAGGAGAWLN LAAAADGEIH EL TRLLEVE GMAQNSMDGM EELRLALATL DPKRVAGGKE TVADWKRRLS RLEAIIQEAQ EESQLQGTLQ DLVTQARGHT DPR QLKIVV EAARGLALGA SAGSQYALLK DKLLRYASAK QSFLAFYETA QPTVFVKHPL TNNLPLLITI SAPPTGWGNG APTR RAQFL AAAGPAKYAG TLWLETESPC DPLNPAYVSA DTQEPLNYIP VYHNFLEYVM PTVLENPEAF SLTPAGRPQA IGPPQ DDQE RRRRTLASVA SARLSAAAAD SYWDTWPDVE SNAGELLREY VSAPKALMED LADNPIVAMT LLAHASLIAS RNHPPY PAP ATDREVILLE QREMMALLVG THPAYAAAFL GAPSFYAGLG LVSALARDGG LGDLLSDSVL TYRLVRSPAS GRGGMPS TT RGSNDGEDAR RLTRHRIAGP PTGFIFFQDA WEEMDTRAAL WPHPEFLGLV HNQSTARARA CMLLLARRCF APEALQQL W HSLRPLEGPV AFQDYLRDFV KQAYTRGEEL PRAEGLEVPR ETPSSYGTVT GRALRNLMPY GTPITGPKRG SGDTIPVSV FEAAVAAAFL GRPLTLFVSS QYLFNLKTLG QVRVVAPLLY CDGHSEPFRS LVETISLNFL QDLDGYSESF EPEMSIFARQ AVWLRELLT EARAAKPKEA RPPTVAILAN RKNIIWKCFT YRHNLPDVQF YFNAAGASRW PTDVLNPSFY EHEDPPLPVG Y QLPPNPRN VQELFSGFPP RVGHGLVSGD GFQSADNTPA SSDRLQQLGG GETDQGEKGS TTAESEASGP PSPQSPLLEK VA PGRPRDW LSPTSSPRDV TVTPGLAAPI TLPGPRLMAR PYFGAETRAS ESPDRSPGSS PRPWPKDSLE LLPQPAPQQP PSS PWASEQ GPIVYTLSPH STPSTASGSQ KKHTIQIPGL VPSQKPSYPP SAPYKPGQST GGIAPTPSAA SLTTFGLQPQ DTQA SSQDP PYGHSIMQRE KKQQGGREEA AEIRPSATRL PTAVGLRPRA PVVAAGAAAS ATPAFDPGEA PSGFPIPQAP ALGSG LAAP AHTPVGALAP RPQKTQAQRP QDAAALPTPT IKAVGARPVP KATGALAAGA RPRGQPTAAP PSAASPPRVS LPVRSR QQQ SPAIPLPPMH SGSEPGARPE VRLSQYRHAG PQTYTVRKEA PPSAASQLPK MPKCKDSMYY PPSGSARYPA PFQALSF SQ SVASPAPSSD QTTLLWNTPS VVTQFLSIED IIREVVTGGS TSGDLVVPSG SPSSLSTAAP EQDLRYSLTL SQASRVLS R FVSQLRRKLE RSTHRLIADL ERLKFLYL UniProtKB: Large tegument protein deneddylase |
-Macromolecule #4: Small capsomere-interacting protein
| Macromolecule | Name: Small capsomere-interacting protein / type: protein_or_peptide / ID: 4 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Epstein-Barr virus (strain B95-8) (Epstein-Barr virus (strain B95-8)) Epstein-Barr virus (strain B95-8) (Epstein-Barr virus (strain B95-8))Strain: B95-8 |
| Molecular weight | Theoretical: 18.1691 KDa |
| Sequence | String: MARRLPKPTL QGRLEADFPD SPLLPKFQEL NQNNLPNDVF REAQRSYLVF LTSQFCYEEY VQRTFGVPRR QRAIDKRQRA SVAGAGAHA HLGGSSATPV QQAQAAASAG TGALASSAPS TAVAQSATPS VSSSISSLRA ATSGATAAAS AAAAVDTGSG G GGQPHDTA PRGARKKQ UniProtKB: Small capsomere-interacting protein |
-Macromolecule #5: Major capsid protein
| Macromolecule | Name: Major capsid protein / type: protein_or_peptide / ID: 5 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Epstein-Barr virus (strain B95-8) (Epstein-Barr virus (strain B95-8)) Epstein-Barr virus (strain B95-8) (Epstein-Barr virus (strain B95-8))Strain: B95-8 |
| Molecular weight | Theoretical: 154.086828 KDa |
| Sequence | String: MASNEGVENR PFPYLTVDAD LLSNLRQSAA EGLFHSFDLL VGKDAREAGI KFEVLLGVYT NAIQYVRFLE TALAVSCVNT EFKDLSRMT DGKIQFRISV PTIAHGDGRR PSKQRTFIVV KNCHKHHIST EMELSMLDLE ILHSIPETPV EYAEYVGAVK T VASALQFG ...String: MASNEGVENR PFPYLTVDAD LLSNLRQSAA EGLFHSFDLL VGKDAREAGI KFEVLLGVYT NAIQYVRFLE TALAVSCVNT EFKDLSRMT DGKIQFRISV PTIAHGDGRR PSKQRTFIVV KNCHKHHIST EMELSMLDLE ILHSIPETPV EYAEYVGAVK T VASALQFG VDALERGLIN TVLSVKLRHA PPMFILQTLA DPTFTERGFS KTVKSDLIAM FKRHLLEHSF FLDRAENMGS GF SQYVRSR LSEMVAAVSG ESVLKGVSTY TTAKGGEPVG GVFIVTDNVL RQLLTFLGEE ADNQIMGPSS YASFVVRGEN LVT AVSYGR VMRTFEHFMA RIVDSPEKAG STKSDLPAVA AGVEDQPRVP ISAAVIKLGN HAVAVESLQK MYNDTQSPYP LNRR MQYSY YFPVGLFMPN PKYTTSAAIK MLDNPTQQLP VEAWIVNKNN LLLAFNLQNA LKVLCHPRLH TPAHTLNSLN AAPAP RDRR ETYSLQHRRP NHMNVLVIVD EFYDNKYAAP VTDIALKCGL PTEDFLHPSN YDLLRLELHP LYDIYIGRDA GERARH RAV HRLMVGNLPT PLAPAAFQEA RGQQFETATS LAHVVDQAVI ETVQDTAYDT AYPAFFYVVE AMIHGFEEKF VMNVPLV SL CINTYWERSG RLAFVNSFSM IKFICRHLGN NAISKEAYSM YRKIYGELIA LEQALMRLAG SDVVGDESVG QYVCALLD P NLLPPVAYTD IFTHLLTVSD RAPQIIIGNE VYADTLAAPQ FIERVGNMDE MAAQFVALYG YRVNGDHDHD FRLHLGPYV DEGHADVLEK IFYYVFLPTC TNAHMCGLGV DFQHVAQTLA YNGPAFSHHF TRDEDILDNL ENGTLRDLLE ISDLRPTVGM IRDLSASFM TCPTFTRAVR VSVDNDVTQQ LAPNPADKRT EQTVLVNGLV AFAFSERTRA VTQCLFHAIP FHMFYGDPRV A ATMHQDVA TFVMRNPQQR AVEAFNRPEQ LFAEYREWHR SPMGKYAAEC LPSLVSISGM TAMHIKMSPM AYIAQAKLKI HP GVAMTVV RTDEILSENI LFSSRASTSM FIGTPNVSRR EARVDAVTFE VHHEMASIDT GLSYSSTMTP ARVAAITTDM GIH TQDFFS VFPAEAFGNQ QVNDYIKAKV GAQRNGTLLR DPRTYLAGMT NVNGAPGLCH GQQATCEIIV TPVTADVAYF QKSN SPRGR AACVVSCENY NQEVAEGLIY DHSRPDAAYE YRSTVNPWAS QLGSLGDIMY NSSYRQTAVP GLYSPCRAFF NKEEL LRNN RGLYNMVNEY SQRLGGHPAT SNTEVQFVVI AGTDVFLEQP CSFLQEAFPA LSASSRALID EFMSVKQTHA PIHYGH YII EEVAPVRRIL KFGNKVVF UniProtKB: Major capsid protein |
-Macromolecule #6: Triplex capsid protein 1
| Macromolecule | Name: Triplex capsid protein 1 / type: protein_or_peptide / ID: 6 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Epstein-Barr virus (strain B95-8) (Epstein-Barr virus (strain B95-8)) Epstein-Barr virus (strain B95-8) (Epstein-Barr virus (strain B95-8))Strain: B95-8 |
| Molecular weight | Theoretical: 39.231539 KDa |
| Sequence | String: MKVQGSVDRR RLQRRIAGLL PPPARRLNIS RGSEFTRDVR GLVEEHAQAS SLSAAAVWRA GLLAPGEVAV AGGGSGGGSF SWSGWRPPV FGDFLIHASS FNNAEATGTP LFQFKQSDPF SGVDAVFTPL SLFILMNHGR GVAARVEAGG GLTRMANLLY D SPATLADL ...String: MKVQGSVDRR RLQRRIAGLL PPPARRLNIS RGSEFTRDVR GLVEEHAQAS SLSAAAVWRA GLLAPGEVAV AGGGSGGGSF SWSGWRPPV FGDFLIHASS FNNAEATGTP LFQFKQSDPF SGVDAVFTPL SLFILMNHGR GVAARVEAGG GLTRMANLLY D SPATLADL VPDFGRLVAD RRFHNFITPV GPLVENIKST YLNKITTVVH GPVVSKAIPR STVKVTVPQE AFVDLDAWLS GG AGGGGGV CFVGGLGLQP CPADARLYVA LTYEEAGPRF TFFQSSRGHC QIMNILRIYY SPSIMHRYAV VQPLHIEELT FGA VACLGT FSATDGWRRS AFNYRGSSLP VVEIDSFYSN VSDWEVIL UniProtKB: Triplex capsid protein 1 |
-Macromolecule #7: Triplex capsid protein 2
| Macromolecule | Name: Triplex capsid protein 2 / type: protein_or_peptide / ID: 7 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Epstein-Barr virus (strain B95-8) (Epstein-Barr virus (strain B95-8)) Epstein-Barr virus (strain B95-8) (Epstein-Barr virus (strain B95-8))Strain: B95-8 |
| Molecular weight | Theoretical: 33.654039 KDa |
| Sequence | String: MDLKVVVSLS SRLYTDEIAK MQQRIGCILP LASTHGTQNV QGLGLGQVYS LETVPDYVSM YNYLSDCTLA VLDEVSVDSL ILTKIVPGQ TYAIKNKYQP FFQWHGTGSL SVMPPVFGRE HATVKLESND VDIVFPMVLP TPIAEEVLQK ILLFNVYSRV V MQAPGNAD ...String: MDLKVVVSLS SRLYTDEIAK MQQRIGCILP LASTHGTQNV QGLGLGQVYS LETVPDYVSM YNYLSDCTLA VLDEVSVDSL ILTKIVPGQ TYAIKNKYQP FFQWHGTGSL SVMPPVFGRE HATVKLESND VDIVFPMVLP TPIAEEVLQK ILLFNVYSRV V MQAPGNAD MLDVHMHLGS VSYLGHHYEL ALPEVPGPLG LALLDNLSLY FCIMVTLLPR ASMRLVRGLI RHEHHDLLNL FQ EMVPDEI ARIDLDDLSV ADDLSRMRVM MTYLQSLASL FNLGPRLATA AYSQETLTAT CWLR UniProtKB: Triplex capsid protein 2 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Average electron dose: 48.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: OTHER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















