+Search query
-Structure paper
| Title | Structure of the human TWIK-2 potassium channel and its inhibition by pimozide. |
|---|---|
| Journal, issue, pages | Proc Natl Acad Sci U S A, Vol. 122, Issue 19, Page e2425709122, Year 2025 |
| Publish date | May 13, 2025 |
 Authors Authors | Nandish K Khanra / Chongyuan Wang / Bryce D Delgado / Stephen B Long /  |
| PubMed Abstract | The potassium channel TWIK-2 is crucial for ATP-induced activation of the NLRP3 inflammasome in macrophages. The channel is a member of the two-pore domain potassium (K2P) channel superfamily and an ...The potassium channel TWIK-2 is crucial for ATP-induced activation of the NLRP3 inflammasome in macrophages. The channel is a member of the two-pore domain potassium (K2P) channel superfamily and an emerging therapeutic target to mitigate severe inflammatory injury involving NLRP3 activation. We report the cryo-EM structure of human TWIK-2. In comparison to other K2P channels, the structure reveals an unusual "up" conformation of Tyr111 in the selectivity filter and a resulting SF1-P1 pocket behind the filter. Density for acyl chains is present in fenestrations within the transmembrane region that connects the central cavity of the pore to the lipid membrane. Despite its importance as a drug target, limited pharmacological tools are available for TWIK-2. A previous study suggested that the FDA-approved small molecule pimozide might inhibit TWIK-2. Using a reconstituted system, we show that pimozide directly inhibits the channel and we determine a cryo-EM structure of a complex with the drug. Pimozide displaces the acyl chains within the fenestrations and binds below the selectivity filter where it would impede ion permeation. The drug may access its binding site by lateral diffusion in the membrane, suggesting that other hydrophobic small molecules could have utility for inhibiting TWIK-2. The work defines the structure of TWIK-2 and provides a structural foundation for development of more specific inhibitors with potential utility as anti-inflammatory drugs. |
 External links External links |  Proc Natl Acad Sci U S A / Proc Natl Acad Sci U S A /  PubMed:40343992 / PubMed:40343992 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 2.85 - 3.17 Å |
| Structure data | EMDB-48216, PDB-9mek: EMDB-48217, PDB-9mel: |
| Chemicals |  ChemComp-K: 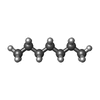 ChemComp-HP6: 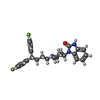 ChemComp-1II: |
| Source |
|
 Keywords Keywords | MEMBRANE PROTEIN / Two-pore potassium channel K2P6 TWIK2 K2P channel / Two-pore potassium channel K2P6 TWIK2 K2P channel pimozide drug NLRP3 inflammasome |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers







 homo sapiens (human)
homo sapiens (human)