+Search query
-Structure paper
| Title | Structural and functional insights of AmpG in muropeptide transport and multiple β-lactam antibiotics resistance. |
|---|---|
| Journal, issue, pages | Nat Commun, Vol. 16, Issue 1, Page 5744, Year 2025 |
| Publish date | Jul 1, 2025 |
 Authors Authors | Nienping Chang / Hoyoung Kim / Uijin Kim / Yongju Cho / Youngki Yoo / Hyunsook Lee / Ji Won Kim / Min Sung Kim / Jaeho Lee / Young-Lag Cho / Kitae Kim / Dongeun Yong / Hyun-Soo Cho /   |
| PubMed Abstract | Anhydromuropeptide permease (AmpG) is a transporter protein located in the inner membrane of certain gram -negative bacteria, involved in peptidoglycan (PG) recycling and β-lactamase induction. ...Anhydromuropeptide permease (AmpG) is a transporter protein located in the inner membrane of certain gram -negative bacteria, involved in peptidoglycan (PG) recycling and β-lactamase induction. Decreased AmpG function reduces resistance of antibiotic-resistant bacteria to β-lactam antibiotics. Therefore, AmpG-targeting inhibitors are promising 'antibiotic adjuvants'. However, as the tertiary structure of AmpG has not yet been identified, the development of targeted inhibitors remains challenging. We present four cryo-electron microscopy (cryo-EM) structures: the apo-inward and apo-outward state structures and the inward-occluded and outward states complexed with the substrate GlcNAc-1,6-anhMurNAc. Through functional analysis and molecular dynamics (MD) simulations, we identified motif A, which stabilizes the outward state, substrate-binding pocket, and protonation-related residues. Based on the structure of AmpG and our experimental results, we propose a muropeptide transport mechanism for AmpG. A deeper understanding of its structure and transport mechanism provides a foundation for the development of antibiotic adjuvants. |
 External links External links |  Nat Commun / Nat Commun /  PubMed:40593790 / PubMed:40593790 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 2.97 - 3.88 Å |
| Structure data | EMDB-39900, PDB-8zbb: EMDB-60093, PDB-8zgz: EMDB-60190, PDB-8zke: EMDB-61285, PDB-9j9z: |
| Chemicals | 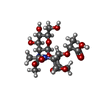 ChemComp-2YP: |
| Source |
|
 Keywords Keywords | PEPTIDE BINDING PROTEIN / transporter / ampg / cryo-em / permease / TRANSPORT PROTEIN / antibiotic resistance / membrane transporter / MEMBRANE PROTEIN / MFS / Anhydromuropeptide permease |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers











 yokenella regensburgei (Enteric Group 45)
yokenella regensburgei (Enteric Group 45) homo sapiens (human)
homo sapiens (human)