+Search query
-Structure paper
| Title | Structures of wild-type and a constitutively closed mutant of connexin26 shed light on channel regulation by CO. |
|---|---|
| Journal, issue, pages | Elife, Vol. 13, Year 2024 |
| Publish date | Jun 3, 2024 |
 Authors Authors | Deborah H Brotherton / Sarbjit Nijjar / Christos G Savva / Nicholas Dale / Alexander David Cameron /  |
| PubMed Abstract | Connexins allow intercellular communication by forming gap junction channels (GJCs) between juxtaposed cells. Connexin26 (Cx26) can be regulated directly by CO. This is proposed to be mediated ...Connexins allow intercellular communication by forming gap junction channels (GJCs) between juxtaposed cells. Connexin26 (Cx26) can be regulated directly by CO. This is proposed to be mediated through carbamylation of K125. We show that mutating K125 to glutamate, mimicking the negative charge of carbamylation, causes Cx26 GJCs to be constitutively closed. Through cryo-EM we observe that the K125E mutation pushes a conformational equilibrium towards the channel having a constricted pore entrance, similar to effects seen on raising the partial pressure of CO. In previous structures of connexins, the cytoplasmic loop, important in regulation and where K125 is located, is disordered. Through further cryo-EM studies we trap distinct states of Cx26 and observe density for the cytoplasmic loop. The interplay between the position of this loop, the conformations of the transmembrane helices and the position of the N-terminal helix, which controls the aperture to the pore, provides a mechanism for regulation. |
 External links External links |  Elife / Elife /  PubMed:38829031 / PubMed:38829031 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 2.1 - 4.9 Å |
| Structure data | EMDB-18290, PDB-8q9z: EMDB-18291, PDB-8qa0: EMDB-18292, PDB-8qa1: EMDB-18293, PDB-8qa2: EMDB-18294, PDB-8qa3:  EMDB-18295: Cryo-EM reconstruction of Cx26 gap junction K125R mutant (D6 symmetry) 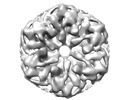 EMDB-18296: Cryo-EM reconstruction of Cx26 gap junction K125E mutant in HEPES buffer  EMDB-18297: Cryo-EM reconstruction of Cx26 gap junction WT in HEPES buffer |
| Chemicals |  ChemComp-LMT:  ChemComp-PTY:  ChemComp-HOH: |
| Source |
|
 Keywords Keywords | MEMBRANE PROTEIN / Gap junction Large Pore Channel Carbon dioxide sensitive |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers









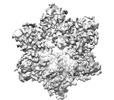



 homo sapiens (human)
homo sapiens (human)