+Search query
-Structure paper
| Title | Structure of PDE3A-SLFN12 complex reveals requirements for activation of SLFN12 RNase. |
|---|---|
| Journal, issue, pages | Nat Commun, Vol. 12, Issue 1, Page 4375, Year 2021 |
| Publish date | Jul 16, 2021 |
 Authors Authors | Colin W Garvie / Xiaoyun Wu / Malvina Papanastasiou / Sooncheol Lee / James Fuller / Gavin R Schnitzler / Steven W Horner / Andrew Baker / Terry Zhang / James P Mullahoo / Lindsay Westlake / Stephanie H Hoyt / Marcus Toetzl / Matthew J Ranaghan / Luc de Waal / Joseph McGaunn / Bethany Kaplan / Federica Piccioni / Xiaoping Yang / Martin Lange / Adrian Tersteegen / Donald Raymond / Timothy A Lewis / Steven A Carr / Andrew D Cherniack / Christopher T Lemke / Matthew Meyerson / Heidi Greulich /   |
| PubMed Abstract | DNMDP and related compounds, or velcrins, induce complex formation between the phosphodiesterase PDE3A and the SLFN12 protein, leading to a cytotoxic response in cancer cells that express elevated ...DNMDP and related compounds, or velcrins, induce complex formation between the phosphodiesterase PDE3A and the SLFN12 protein, leading to a cytotoxic response in cancer cells that express elevated levels of both proteins. The mechanisms by which velcrins induce complex formation, and how the PDE3A-SLFN12 complex causes cancer cell death, are not fully understood. Here, we show that PDE3A and SLFN12 form a heterotetramer stabilized by binding of DNMDP. Interactions between the C-terminal alpha helix of SLFN12 and residues near the active site of PDE3A are required for complex formation, and are further stabilized by interactions between SLFN12 and DNMDP. Moreover, we demonstrate that SLFN12 is an RNase, that PDE3A binding increases SLFN12 RNase activity, and that SLFN12 RNase activity is required for DNMDP response. This new mechanistic understanding will facilitate development of velcrin compounds into new cancer therapies. |
 External links External links |  Nat Commun / Nat Commun /  PubMed:34272366 / PubMed:34272366 /  PubMed Central PubMed Central |
| Methods | EM (single particle) / X-ray diffraction |
| Resolution | 1.7 - 3.22 Å |
| Structure data | EMDB-23494, PDB-7lrc: EMDB-23495, PDB-7lrd: EMDB-23496, PDB-7lre:  PDB-7kwe:  PDB-7l27:  PDB-7l28:  PDB-7l29: |
| Chemicals | 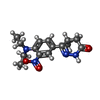 ChemComp-X5M:  ChemComp-MN:  ChemComp-MG:  ChemComp-ACT:  ChemComp-HOH:  ChemComp-CA: 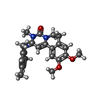 ChemComp-XKG:  ChemComp-AMP:  ChemComp-ZN: |
| Source |
|
 Keywords Keywords | HYDROLASE / HYDROLASE/HYDROLASE INHIBITOR / HYDROLASE-HYDROLASE INHIBITOR complex / complex / velcrin / molecular glue / DNMDP / RNA BINDING PROTEIN |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers









 homo sapiens (human)
homo sapiens (human)