+Search query
-Structure paper
| Title | A hybrid approach reveals the allosteric regulation of GTP cyclohydrolase I. |
|---|---|
| Journal, issue, pages | Proc Natl Acad Sci U S A, Vol. 117, Issue 50, Page 31838-31849, Year 2020 |
| Publish date | Dec 15, 2020 |
 Authors Authors | Rebecca Ebenhoch / Simone Prinz / Susann Kaltwasser / Deryck J Mills / Robert Meinecke / Martin Rübbelke / Dirk Reinert / Margit Bauer / Lisa Weixler / Markus Zeeb / Janet Vonck / Herbert Nar /  |
| PubMed Abstract | Guanosine triphosphate (GTP) cyclohydrolase I (GCH1) catalyzes the conversion of GTP to dihydroneopterin triphosphate (H2NTP), the initiating step in the biosynthesis of tetrahydrobiopterin (BH4). ...Guanosine triphosphate (GTP) cyclohydrolase I (GCH1) catalyzes the conversion of GTP to dihydroneopterin triphosphate (H2NTP), the initiating step in the biosynthesis of tetrahydrobiopterin (BH4). Besides other roles, BH4 functions as cofactor in neurotransmitter biosynthesis. The BH4 biosynthetic pathway and GCH1 have been identified as promising targets to treat pain disorders in patients. The function of mammalian GCH1s is regulated by a metabolic sensing mechanism involving a regulator protein, GCH1 feedback regulatory protein (GFRP). GFRP binds to GCH1 to form inhibited or activated complexes dependent on availability of cofactor ligands, BH4 and phenylalanine, respectively. We determined high-resolution structures of human GCH1-GFRP complexes by cryoelectron microscopy (cryo-EM). Cryo-EM revealed structural flexibility of specific and relevant surface lining loops, which previously was not detected by X-ray crystallography due to crystal packing effects. Further, we studied allosteric regulation of isolated GCH1 by X-ray crystallography. Using the combined structural information, we are able to obtain a comprehensive picture of the mechanism of allosteric regulation. Local rearrangements in the allosteric pocket upon BH4 binding result in drastic changes in the quaternary structure of the enzyme, leading to a more compact, tense form of the inhibited protein, and translocate to the active site, leading to an open, more flexible structure of its surroundings. Inhibition of the enzymatic activity is not a result of hindrance of substrate binding, but rather a consequence of accelerated substrate binding kinetics as shown by saturation transfer difference NMR (STD-NMR) and site-directed mutagenesis. We propose a dissociation rate controlled mechanism of allosteric, noncompetitive inhibition. |
 External links External links |  Proc Natl Acad Sci U S A / Proc Natl Acad Sci U S A /  PubMed:33229582 / PubMed:33229582 /  PubMed Central PubMed Central |
| Methods | EM (single particle) / X-ray diffraction |
| Resolution | 1.726 - 3.0 Å |
| Structure data | EMDB-11113, PDB-6z80: EMDB-11114, PDB-6z85:  PDB-6z86:  PDB-6z87:  PDB-6z88:  PDB-6z89:  PDB-7acc:  PDB-7al9:  PDB-7ala:  PDB-7alb:  PDB-7alc: |
| Chemicals |  ChemComp-ZN:  ChemComp-PHE:  ChemComp-8GT: 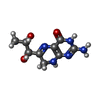 ChemComp-HBI: 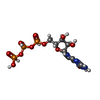 ChemComp-QBQ:  ChemComp-HOH: 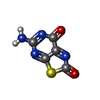 ChemComp-QBK:  ChemComp-K: 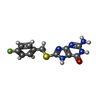 ChemComp-5RW:  ChemComp-NA: |
| Source |
|
 Keywords Keywords | HYDROLASE / GTP cyclohydrolase GFTP / I / EC:3.5.4.16 / Tetrahydrobiopterin (BH4) synthesis / Cytosol / Zinc Ion Binding / Hydrolase Activity / Metal Ion Binding / Nucleotide Binding / allosteric enzyme / substrate analogue bound / allosteric inhibited / GTP cyclohydrolase I / allosteric inhibitor / PROTEIN BINDING / GTP cyclohydrolase 1 feedback regulatory protein / regulatory protein / L-phenylalanine binding (Phe) synthesis allosteric regulation / GTP cyclohydrolase 1 / GTPCH1 / GTP cyclohydrolase I regulatory protein / GFRP / allosteric regulation / complex / allosteric regulator |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers







 homo sapiens (human)
homo sapiens (human)