+Search query
-Structure paper
| Title | Covalent modifications of the active site cysteine occur as a result of mutating the glutamate of the catalytic triad in the amidase from Nesterenkonia sp. |
|---|---|
| Journal, issue, pages | To be Published |
| Publish date | Jan 30, 2013 (structure data deposition date) |
 Authors Authors | Kimani, S.W. / Hunter, R. / Vlok, M. / Watermeyer, J. / Sewell, B.T. |
 External links External links | Search PubMed |
| Methods | X-ray diffraction |
| Resolution | 1.4 - 1.92 Å |
| Structure data |  PDB-4izs:  PDB-4izt:  PDB-4izu:  PDB-4izv:  PDB-4izw: |
| Chemicals |  ChemComp-PEG: 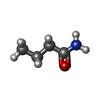 ChemComp-BMD:  ChemComp-HOH: 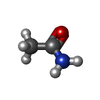 ChemComp-ACM: 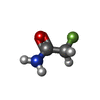 ChemComp-FTM: 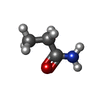 ChemComp-ROP:  ChemComp-1HC: |
| Source |
|
 Keywords Keywords | HYDROLASE / butyramide / hydrolase/substrate / fluoroacetamide / acetamide / hydrolase-substrate complex / propionamide / acrylamide (prop-2-enamide) / cysteine 145 / active site |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers



 nesterenkonia sp. 10004 (bacteria)
nesterenkonia sp. 10004 (bacteria)