+検索条件
-Structure paper
| タイトル | Structural basis for anaerobic alkane activation by a multisubunit glycyl radical enzyme. |
|---|---|
| ジャーナル・号・ページ | Proc Natl Acad Sci U S A, Vol. 122, Issue 32, Page e2510389122, Year 2025 |
| 掲載日 | 2025年8月12日 |
 著者 著者 | Mary C Andorfer / Talya S Levitz / Jian Liu / Ankush Chakraborty / Devin T King-Roberts / Delight Nweneka / Christa N Imrich / Catherine L Drennan /  |
| PubMed 要旨 | X-succinate synthases (XSSs) are glycyl radical enzymes (GREs) that catalyze the addition of hydrocarbons to fumarate via radical chemistry, thereby activating them for microbial metabolism. To date, ...X-succinate synthases (XSSs) are glycyl radical enzymes (GREs) that catalyze the addition of hydrocarbons to fumarate via radical chemistry, thereby activating them for microbial metabolism. To date, the only structurally characterized XSS is benzylsuccinate synthase (BSS), which functionalizes toluene. A distinct subclass of XSSs acts on saturated hydrocarbons, which possess much stronger C(sp)-H bonds than toluene, suggesting mechanistic and structural differences from BSS. Here, we use cryogenic electron microscopy to determine the structure of one such enzyme, (1-methylalkyl)succinate synthase (MASS) from strain HxN1, which functionalizes -alkanes (C6-C8). The structure reveals an asymmetric dimer in which both sides contain a catalytic α-subunit and accessory γ-subunit. One α-subunit also binds two additional subunits, β and δ. The β-subunit binds a [4Fe-4S] cluster and adopts a fold similar to BSSβ. The β-subunit appears to regulate the flexibility of the α-subunit to enable opening of the active site, affording the binding of -alkane substrates. The δ-subunit, which lacks homology to known GRE subunits, adopts a rubredoxin-like fold that binds a single Fe ion, an architecture not previously reported for GREs. MASSδ occupies the same region of the α-subunit as the activating enzyme (AE) and may regulate the conformational changes required for glycyl radical installation. Structural comparisons between MASS and BSS reveal differences in how fumarate is bound and show amino acid substitutions that could account for the binding of alkanes versus toluene. Together, this structure offers insight into anaerobic alkane activation via fumarate addition. |
 リンク リンク |  Proc Natl Acad Sci U S A / Proc Natl Acad Sci U S A /  PubMed:40758891 / PubMed:40758891 /  PubMed Central PubMed Central |
| 手法 | EM (単粒子) |
| 解像度 | 2.8 Å |
| 構造データ | EMDB-70238, PDB-9o8u: |
| 化合物 | 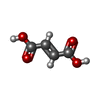 ChemComp-FUM: 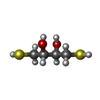 ChemComp-DTT:  ChemComp-SF4:  ChemComp-FE: |
| 由来 |
|
 キーワード キーワード | LYASE / glycyl radical enzyme / (1-methylalkyl)succinate synthase / X-succinate synthase / alkylsuccinate synthase / Hydrocarbon degradation / fumarate addition |
 ムービー
ムービー コントローラー
コントローラー 構造ビューア
構造ビューア 万見文献について
万見文献について





 azoarcus sp. hxn1 (バクテリア)
azoarcus sp. hxn1 (バクテリア)