+Search query
-Structure paper
| Title | Structures of myxobacterial phytochrome revealed by cryo-EM using the Spotiton technique and with x-ray crystallography. |
|---|---|
| Journal, issue, pages | Struct Dyn, Vol. 12, Issue 3, Page 034701, Year 2025 |
| Publish date | May 1, 2025 |
 Authors Authors | Prabin Karki / David Menendez / William Budell / Shishir Dangi / Carolina Hernandez / Joshua Mendez / Srinivasan Muniyappan / Shibom Basu / Peter Schwander / Tek N Malla / Emina A Stojković / Marius Schmidt /   |
| PubMed Abstract | Phytochromes are red-light photoreceptors first identified in plants, with homologs found in bacteria and fungi, that regulate a variety of critical physiological processes. They undergo a reversible ...Phytochromes are red-light photoreceptors first identified in plants, with homologs found in bacteria and fungi, that regulate a variety of critical physiological processes. They undergo a reversible photocycle between two distinct states: a red-light-absorbing Pr form and a far-red light-absorbing Pfr form. This Pr/Pfr photoconversion controls the activity of a C-terminal enzymatic domain, typically a histidine kinase (HK). However, the molecular mechanisms underlying light-induced regulation of HK activity in bacteria remain poorly understood, as only a few structures of unmodified bacterial phytochromes with HK activity are known. Recently, cryo-EM structures of a wild-type bacterial phytochrome with HK activity are solved that reveal homodimers in both the Pr and Pfr states, as well as a heterodimer with individual monomers in distinct Pr and Pfr states. Cryo-EM structures of a truncated version of the same phytochrome-lacking the HK domain-also show a homodimer in the Pfr state and a Pr/Pfr heterodimer. Here, we describe in detail how structural information is obtained from cryo-EM data on a full-length intact bacteriophytochrome, and how the cryo-EM structure can contribute to the understanding of the function of the phytochrome. In addition, we compare the cryo-EM structure to an unusual x-ray structure that is obtained from a fragmented full-length phytochrome crystallized in the Pr-state. |
 External links External links |  Struct Dyn / Struct Dyn /  PubMed:40322674 / PubMed:40322674 /  PubMed Central PubMed Central |
| Methods | EM (single particle) / X-ray diffraction |
| Resolution | 2.1 - 6.0 Å |
| Structure data |  EMDB-70119: Pr/Pr homodimer of Stigmatella aurantiaca bacteriophytochrome 2  PDB-9naa: |
| Chemicals | 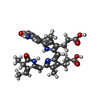 ChemComp-EL5:  ChemComp-HOH: |
| Source |
|
 Keywords Keywords | SIGNALING PROTEIN / phytochrome / myxobacteria / biliverdin |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers



 stigmatella aurantiaca (bacteria)
stigmatella aurantiaca (bacteria)