+検索条件
-Structure paper
| タイトル | Structure of the GTP Form of Elongation Factor 4 (EF4) Bound to the Ribosome. |
|---|---|
| ジャーナル・号・ページ | J Biol Chem, Vol. 291, Issue 25, Page 12943-12950, Year 2016 |
| 掲載日 | 2016年6月17日 |
 著者 著者 | Veerendra Kumar / Rya Ero / Tofayel Ahmed / Kwok Jian Goh / Yin Zhan / Shashi Bhushan / Yong-Gui Gao /  |
| PubMed 要旨 | Elongation factor 4 (EF4) is a member of the family of ribosome-dependent translational GTPase factors, along with elongation factor G and BPI-inducible protein A. Although EF4 is highly conserved in ...Elongation factor 4 (EF4) is a member of the family of ribosome-dependent translational GTPase factors, along with elongation factor G and BPI-inducible protein A. Although EF4 is highly conserved in bacterial, mitochondrial, and chloroplast genomes, its exact biological function remains controversial. Here we present the cryo-EM reconstitution of the GTP form of EF4 bound to the ribosome with P and E site tRNAs at 3.8-Å resolution. Interestingly, our structure reveals an unrotated ribosome rather than a clockwise-rotated ribosome, as observed in the presence of EF4-GDP and P site tRNA. In addition, we also observed a counterclockwise-rotated form of the above complex at 5.7-Å resolution. Taken together, our results shed light on the interactions formed between EF4, the ribosome, and the P site tRNA and illuminate the GTPase activation mechanism at previously unresolved detail. |
 リンク リンク |  J Biol Chem / J Biol Chem /  PubMed:27137929 / PubMed:27137929 /  PubMed Central PubMed Central |
| 手法 | EM (単粒子) |
| 解像度 | 3.8 - 5.9 Å |
| 構造データ | |
| 化合物 | 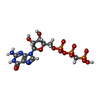 ChemComp-GCP: |
| 由来 |
|
 キーワード キーワード | RIBOSOME / EF4/LepA / translational GTPase factors / translocation / reverse |
 ムービー
ムービー コントローラー
コントローラー 構造ビューア
構造ビューア 万見文献について
万見文献について








 Thermus thermophilus (バクテリア)
Thermus thermophilus (バクテリア)