+Search query
-Structure paper
| Title | Molecular basis of convergent evolution of ACE2 receptor utilization among HKU5 coronaviruses. |
|---|---|
| Journal, issue, pages | Cell, Vol. 188, Issue 6, Page 1711-1728.e21, Year 2025 |
| Publish date | Mar 20, 2025 |
 Authors Authors | Young-Jun Park / Chen Liu / Jimin Lee / Jack T Brown / Cheng-Bao Ma / Peng Liu / Risako Gen / Qing Xiong / Samantha K Zepeda / Cameron Stewart / Amin Addetia / Caroline J Craig / M Alejandra Tortorici / Abeer N Alshukairi / Tyler N Starr / Huan Yan / David Veesler /    |
| PubMed Abstract | DPP4 was considered a canonical receptor for merbecoviruses until the recent discovery of African bat-borne MERS-related coronaviruses using ACE2. The extent and diversity of ACE2 utilization among ...DPP4 was considered a canonical receptor for merbecoviruses until the recent discovery of African bat-borne MERS-related coronaviruses using ACE2. The extent and diversity of ACE2 utilization among merbecoviruses and their receptor species tropism remain unknown. Here, we reveal that HKU5 enters host cells utilizing Pipistrellus abramus (P.abr) and several non-bat mammalian ACE2s through a binding mode distinct from that of any other known ACE2-using coronaviruses. We defined the molecular determinants of receptor species tropism and identified a single amino acid mutation enabling HKU5 to utilize human ACE2, providing proof of principle for machine-learning-assisted outbreak preparedness. We show that MERS-CoV and HKU5 have markedly distinct antigenicity and identified several HKU5 inhibitors, including two clinical compounds. Our findings profoundly alter our understanding of coronavirus evolution, as several merbecovirus clades independently evolved ACE2 utilization, and pave the way for developing countermeasures against viruses poised for human emergence. |
 External links External links |  Cell / Cell /  PubMed:39922192 / PubMed:39922192 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 2.0 - 3.1 Å |
| Structure data | EMDB-46512, PDB-9d32: EMDB-47358, PDB-9e0i: EMDB-47823, PDB-9ea0: EMDB-48048, PDB-9eh8: |
| Chemicals |  ChemComp-NAG:  ChemComp-ZN:  ChemComp-HOH: 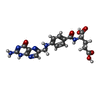 ChemComp-FOL:  ChemComp-EIC: |
| Source |
|
 Keywords Keywords | VIRAL PROTEIN/HYDROLASE / MERS-related HKU5 coronaviruses / MERSr-CoV / Spike glycoprotein / fusion protein / Pipistrellus abramus ACE2 / Structural Genomics / Seattle Structural Genomics Center for Infectious Disease / SSGCID / inhibitor / VIRAL PROTEIN-HYDROLASE complex / VIRAL PROTEIN / VIRAL PROTEIN/IMMUNE SYSTEM / VIRAL PROTEIN-IMMUNE SYSTEM complex |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers











 Homo sapiens (human)
Homo sapiens (human) pipistrellus abramus (Japanese house bat)
pipistrellus abramus (Japanese house bat) pipistrellus bat coronavirus hku5
pipistrellus bat coronavirus hku5