+Search query
-Structure paper
| Title | Binding adaptability of chemical ligands to polymorphic α-synuclein amyloid fibrils. |
|---|---|
| Journal, issue, pages | Proc Natl Acad Sci U S A, Vol. 121, Issue 35, Page e2321633121, Year 2024 |
| Publish date | Aug 27, 2024 |
 Authors Authors | Kaien Liu / Youqi Tao / Qinyue Zhao / Wencheng Xia / Xiang Li / Shenqing Zhang / Yuxuan Yao / Huaijiang Xiang / Chao Han / Li Tan / Bo Sun / Dan Li / Ang Li / Cong Liu /  |
| PubMed Abstract | α-synuclein (α-syn) assembles into structurally distinct fibril polymorphs seen in different synucleinopathies, such as Parkinson's disease and multiple system atrophy. Targeting these unique ...α-synuclein (α-syn) assembles into structurally distinct fibril polymorphs seen in different synucleinopathies, such as Parkinson's disease and multiple system atrophy. Targeting these unique fibril structures using chemical ligands holds diagnostic significance for different disease subtypes. However, the molecular mechanisms governing small molecules interacting with different fibril polymorphs remain unclear. Here, we investigated the interactions of small molecules belonging to four distinct scaffolds, with different α-syn fibril polymorphs. Using cryo-electron microscopy, we determined the structures of these molecules when bound to the fibrils formed by E46K mutant α-syn and compared them to those bound with wild-type α-syn fibrils. Notably, we observed that these ligands exhibit remarkable binding adaptability, as they engage distinct binding sites across different fibril polymorphs. While the molecular scaffold primarily steered the binding locations and geometries on specific sites, the conjugated functional groups further refined this adaptable binding by fine-tuning the geometries and binding sites. Overall, our finding elucidates the adaptability of small molecules binding to different fibril structures, which sheds light on the diagnostic tracer and drug developments tailored to specific pathological fibril polymorphs. |
 External links External links |  Proc Natl Acad Sci U S A / Proc Natl Acad Sci U S A /  PubMed:39172784 / PubMed:39172784 /  PubMed Central PubMed Central |
| Methods | EM (helical sym.) |
| Resolution | 2.7 - 3.5 Å |
| Structure data | EMDB-38097, PDB-8x7b: EMDB-38103, PDB-8x7l: EMDB-38104, PDB-8x7m: EMDB-38105, PDB-8x7o: EMDB-38106, PDB-8x7p: EMDB-38107, PDB-8x7q: EMDB-38108, PDB-8x7r: EMDB-60226, PDB-8zli: EMDB-60231, PDB-8zlo: EMDB-60232, PDB-8zlp: EMDB-60262, PDB-8zmy: |
| Chemicals |  ChemComp-TFX: 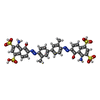 ChemComp-IZ8: 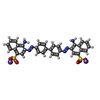 ChemComp-CGO:  ChemComp-IZV:  ChemComp-V79:  ChemComp-3LS: 
ChemComp-Y9W:  PDB-1l13:  ChemComp-1KI: |
| Source |
|
 Keywords Keywords | PROTEIN FIBRIL / amyloid fibril / complex |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers
























 homo sapiens (human)
homo sapiens (human)