+Search query
-Structure paper
| Title | Positive allosteric mechanisms of adenosine A receptor-mediated analgesia. |
|---|---|
| Journal, issue, pages | Nature, Vol. 597, Issue 7877, Page 571-576, Year 2021 |
| Publish date | Sep 8, 2021 |
 Authors Authors | Christopher J Draper-Joyce / Rebecca Bhola / Jinan Wang / Apurba Bhattarai / Anh T N Nguyen / India Cowie-Kent / Kelly O'Sullivan / Ling Yeong Chia / Hariprasad Venugopal / Celine Valant / David M Thal / Denise Wootten / Nicolas Panel / Jens Carlsson / Macdonald J Christie / Paul J White / Peter Scammells / Lauren T May / Patrick M Sexton / Radostin Danev / Yinglong Miao / Alisa Glukhova / Wendy L Imlach / Arthur Christopoulos /     |
| PubMed Abstract | The adenosine A receptor (AR) is a promising therapeutic target for non-opioid analgesic agents to treat neuropathic pain. However, development of analgesic orthosteric AR agonists has failed because ...The adenosine A receptor (AR) is a promising therapeutic target for non-opioid analgesic agents to treat neuropathic pain. However, development of analgesic orthosteric AR agonists has failed because of a lack of sufficient on-target selectivity as well as off-tissue adverse effects. Here we show that [2-amino-4-(3,5-bis(trifluoromethyl)phenyl)thiophen-3-yl)(4-chlorophenyl)methanone] (MIPS521), a positive allosteric modulator of the AR, exhibits analgesic efficacy in rats in vivo through modulation of the increased levels of endogenous adenosine that occur in the spinal cord of rats with neuropathic pain. We also report the structure of the AR co-bound to adenosine, MIPS521 and a G heterotrimer, revealing an extrahelical lipid-detergent-facing allosteric binding pocket that involves transmembrane helixes 1, 6 and 7. Molecular dynamics simulations and ligand kinetic binding experiments support a mechanism whereby MIPS521 stabilizes the adenosine-receptor-G protein complex. This study provides proof of concept for structure-based allosteric drug design of non-opioid analgesic agents that are specific to disease contexts. |
 External links External links |  Nature / Nature /  PubMed:34497422 / PubMed:34497422 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 3.2 - 3.3 Å |
| Structure data | EMDB-23280, PDB-7ld3: EMDB-23281, PDB-7ld4: |
| Chemicals | 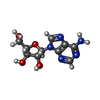 ChemComp-ADN: 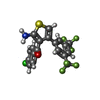 ChemComp-XTD:  ChemComp-HOH: |
| Source |
|
 Keywords Keywords | SIGNALING PROTEIN / membrane protein / active-state G protein-coupled receptor / adenosine A1 receptor / PAM / allosteric modulator |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers







 homo sapiens (human)
homo sapiens (human)