+Search query
-Structure paper
| Title | Structural basis for human mitochondrial tRNA maturation. |
|---|---|
| Journal, issue, pages | Nat Commun, Vol. 15, Issue 1, Page 4683, Year 2024 |
| Publish date | Jun 1, 2024 |
 Authors Authors | Vincent Meynier / Steven W Hardwick / Marjorie Catala / Johann J Roske / Stephanie Oerum / Dimitri Y Chirgadze / Pierre Barraud / Wyatt W Yue / Ben F Luisi / Carine Tisné /   |
| PubMed Abstract | The human mitochondrial genome is transcribed into two RNAs, containing mRNAs, rRNAs and tRNAs, all dedicated to produce essential proteins of the respiratory chain. The precise excision of tRNAs by ...The human mitochondrial genome is transcribed into two RNAs, containing mRNAs, rRNAs and tRNAs, all dedicated to produce essential proteins of the respiratory chain. The precise excision of tRNAs by the mitochondrial endoribonucleases (mt-RNase), P and Z, releases all RNA species from the two RNA transcripts. The tRNAs then undergo 3'-CCA addition. In metazoan mitochondria, RNase P is a multi-enzyme assembly that comprises the endoribonuclease PRORP and a tRNA methyltransferase subcomplex. The requirement for this tRNA methyltransferase subcomplex for mt-RNase P cleavage activity, as well as the mechanisms of pre-tRNA 3'-cleavage and 3'-CCA addition, are still poorly understood. Here, we report cryo-EM structures that visualise four steps of mitochondrial tRNA maturation: 5' and 3' tRNA-end processing, methylation and 3'-CCA addition, and explain the defined sequential order of the tRNA processing steps. The methyltransferase subcomplex recognises the pre-tRNA in a distinct mode that can support tRNA-end processing and 3'-CCA addition, likely resulting from an evolutionary adaptation of mitochondrial tRNA maturation complexes to the structurally-fragile mitochondrial tRNAs. This subcomplex can also ensure a tRNA-folding quality-control checkpoint before the sequential docking of the maturation enzymes. Altogether, our study provides detailed molecular insight into RNA-transcript processing and tRNA maturation in human mitochondria. |
 External links External links |  Nat Commun / Nat Commun /  PubMed:38824131 / PubMed:38824131 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 2.47 - 4.5 Å |
| Structure data | EMDB-16543, PDB-8cbk: EMDB-16544, PDB-8cbl: EMDB-16545, PDB-8cbm: EMDB-16547, PDB-8cbo:  EMDB-16667: Consensus Refinement - Map 1  EMDB-16668: Local refinement - Map 2  EMDB-16669: Cryo-EM structure of human mitochondrial RNase P in complex with mitochondrial pre-tRNA-His(5,Ser)  EMDB-16673: Consensus Refinement - Map 1  EMDB-16674: Local refinement - Map 2 (mask 1)  EMDB-16675: Local refinement - map 3 (Mask 2)  EMDB-16690: Consensus Refinement - Map 1  EMDB-16691: Local refinement - Map 2 (Mask 1)  EMDB-16692: Global Refinement - Map 1 |
| Chemicals |  ChemComp-NAD:  ChemComp-ZN:  ChemComp-SAH:  ChemComp-MG:  ChemComp-HOH: 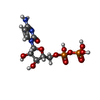 ChemComp-CDP: |
| Source |
|
 Keywords Keywords | RNA BINDING PROTEIN / RNA maturation / RNA modification / mitochondrial tRNA / RNA methyltransferase / MRPP1 / MRPP2 / MRPP3 / m6A / RNA binding / RNA recognition |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers











 homo sapiens (human)
homo sapiens (human)