[English] 日本語
 Yorodumi
Yorodumi- EMDB-16547: Structure of human mitochondrial MRPP1-MRPP2 in complex with mito... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of human mitochondrial MRPP1-MRPP2 in complex with mitochondrial pre-tRNA-Ile | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | RNA maturation / RNA modification / mitochondrial tRNA / RNA methyltransferase / MRPP1 / MRPP2 / MRPP3 / RNA binding protein / m6A / RNA binding / RNA recognition | |||||||||
| Function / homology |  Function and homology information Function and homology informationbrexanolone metabolic process / isoursodeoxycholate 7-beta-dehydrogenase (NAD+) activity / ursodeoxycholate 7-beta-dehydrogenase (NAD+) activity / 3-hydroxy-2-methylbutyryl-CoA dehydrogenase / 3-hydroxy-2-methylbutyryl-CoA dehydrogenase activity / mitochondrial tRNA methylation / tRNA (adenine9-N1)-methyltransferase / mitochondrial tRNA processing / tRNA (adenine(9)-N1)-methyltransferase activity / mitochondrial ribonuclease P complex ...brexanolone metabolic process / isoursodeoxycholate 7-beta-dehydrogenase (NAD+) activity / ursodeoxycholate 7-beta-dehydrogenase (NAD+) activity / 3-hydroxy-2-methylbutyryl-CoA dehydrogenase / 3-hydroxy-2-methylbutyryl-CoA dehydrogenase activity / mitochondrial tRNA methylation / tRNA (adenine9-N1)-methyltransferase / mitochondrial tRNA processing / tRNA (adenine(9)-N1)-methyltransferase activity / mitochondrial ribonuclease P complex / mitochondrial tRNA 5'-end processing / chenodeoxycholate 7-alpha-dehydrogenase (NAD+) activity / tRNA modification in the mitochondrion / rRNA processing in the mitochondrion / tRNA processing in the mitochondrion / tRNA (guanine9-N1)-methyltransferase / tRNA (guanosine(9)-N1)-methyltransferase activity / mitochondrial tRNA 3'-end processing / 7alpha-hydroxysteroid dehydrogenase / 17-beta-hydroxysteroid dehydrogenase (NAD+) activity / cholate 7-alpha-dehydrogenase (NAD+) activity / mitochondrial RNA 5'-end processing / C21-steroid hormone metabolic process / tRNA methyltransferase complex / 3-hydroxyacyl-CoA dehydrogenase / L-isoleucine catabolic process / 3alpha(17beta)-hydroxysteroid dehydrogenase (NAD+) / (3S)-3-hydroxyacyl-CoA dehydrogenase (NAD+) activity / : / 3alpha(or 20beta)-hydroxysteroid dehydrogenase / androstan-3-alpha,17-beta-diol dehydrogenase (NAD+) activity / testosterone dehydrogenase (NAD+) activity / positive regulation of mitochondrial translation / bile acid biosynthetic process / 17beta-estradiol 17-dehydrogenase / estradiol 17-beta-dehydrogenase [NAD(P)+] activity / Branched-chain amino acid catabolism / estrogen metabolic process / fatty acid beta-oxidation / androgen metabolic process / mitochondrial nucleoid / Mitochondrial protein degradation / Transferases; Transferring one-carbon groups; Methyltransferases / mitochondrion organization / fatty acid metabolic process / lipid metabolic process / mRNA processing / protein homotetramerization / tRNA binding / mitochondrial matrix / mitochondrion / RNA binding / nucleoplasm / identical protein binding / nucleus / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
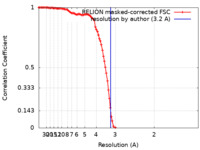
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | MEYNIER V / HARDWICK S / CATALA M / ROSKE J / OERUM S / CHIRGADZE D / BARRAUD P / LUISI B / TISNE C | |||||||||
| Funding support |  France, 1 items France, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structural basis for human mitochondrial tRNA maturation. Authors: Vincent Meynier / Steven W Hardwick / Marjorie Catala / Johann J Roske / Stephanie Oerum / Dimitri Y Chirgadze / Pierre Barraud / Wyatt W Yue / Ben F Luisi / Carine Tisné /   Abstract: The human mitochondrial genome is transcribed into two RNAs, containing mRNAs, rRNAs and tRNAs, all dedicated to produce essential proteins of the respiratory chain. The precise excision of tRNAs by ...The human mitochondrial genome is transcribed into two RNAs, containing mRNAs, rRNAs and tRNAs, all dedicated to produce essential proteins of the respiratory chain. The precise excision of tRNAs by the mitochondrial endoribonucleases (mt-RNase), P and Z, releases all RNA species from the two RNA transcripts. The tRNAs then undergo 3'-CCA addition. In metazoan mitochondria, RNase P is a multi-enzyme assembly that comprises the endoribonuclease PRORP and a tRNA methyltransferase subcomplex. The requirement for this tRNA methyltransferase subcomplex for mt-RNase P cleavage activity, as well as the mechanisms of pre-tRNA 3'-cleavage and 3'-CCA addition, are still poorly understood. Here, we report cryo-EM structures that visualise four steps of mitochondrial tRNA maturation: 5' and 3' tRNA-end processing, methylation and 3'-CCA addition, and explain the defined sequential order of the tRNA processing steps. The methyltransferase subcomplex recognises the pre-tRNA in a distinct mode that can support tRNA-end processing and 3'-CCA addition, likely resulting from an evolutionary adaptation of mitochondrial tRNA maturation complexes to the structurally-fragile mitochondrial tRNAs. This subcomplex can also ensure a tRNA-folding quality-control checkpoint before the sequential docking of the maturation enzymes. Altogether, our study provides detailed molecular insight into RNA-transcript processing and tRNA maturation in human mitochondria. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16547.map.gz emd_16547.map.gz | 98.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16547-v30.xml emd-16547-v30.xml emd-16547.xml emd-16547.xml | 25.4 KB 25.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_16547_fsc.xml emd_16547_fsc.xml | 11.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_16547.png emd_16547.png | 62.2 KB | ||
| Filedesc metadata |  emd-16547.cif.gz emd-16547.cif.gz | 7.2 KB | ||
| Others |  emd_16547_half_map_1.map.gz emd_16547_half_map_1.map.gz emd_16547_half_map_2.map.gz emd_16547_half_map_2.map.gz | 98.5 MB 98.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16547 http://ftp.pdbj.org/pub/emdb/structures/EMD-16547 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16547 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16547 | HTTPS FTP |
-Validation report
| Summary document |  emd_16547_validation.pdf.gz emd_16547_validation.pdf.gz | 697 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_16547_full_validation.pdf.gz emd_16547_full_validation.pdf.gz | 696.5 KB | Display | |
| Data in XML |  emd_16547_validation.xml.gz emd_16547_validation.xml.gz | 18.9 KB | Display | |
| Data in CIF |  emd_16547_validation.cif.gz emd_16547_validation.cif.gz | 24.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16547 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16547 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16547 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16547 | HTTPS FTP |
-Related structure data
| Related structure data |  8cboMC  8cbkC  8cblC  8cbmC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_16547.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16547.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.723 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_16547_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_16547_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Human mitochondrial MRPP1-MRPP2 in complex with mitochondrial pre...
| Entire | Name: Human mitochondrial MRPP1-MRPP2 in complex with mitochondrial pre-tRNAIle |
|---|---|
| Components |
|
-Supramolecule #1: Human mitochondrial MRPP1-MRPP2 in complex with mitochondrial pre...
| Supramolecule | Name: Human mitochondrial MRPP1-MRPP2 in complex with mitochondrial pre-tRNAIle type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Molecular weight | Theoretical: 170 KDa |
-Supramolecule #2: Human mitochondrial MRPP1-MRPP2
| Supramolecule | Name: Human mitochondrial MRPP1-MRPP2 / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #3: Mitochondrial pre-tRNAIle
| Supramolecule | Name: Mitochondrial pre-tRNAIle / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: 3-hydroxyacyl-CoA dehydrogenase type-2
| Macromolecule | Name: 3-hydroxyacyl-CoA dehydrogenase type-2 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO / EC number: 3-hydroxyacyl-CoA dehydrogenase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 26.342254 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SVKGLVAVIT GGASGLGLAT AERLVGQGAS AVLLDLPNSG GEAQAKKLGN NCVFAPADVT SEKDVQTALA LAKGKFGRVD VAVNCAGIA VASKTYNLKK GQTHTLEDFQ RVLDVNLMGT FNVIRLVAGE MGQNEPDQGG QRGVIINTAS VAAFEGQVGQ A AYSASKGG ...String: SVKGLVAVIT GGASGLGLAT AERLVGQGAS AVLLDLPNSG GEAQAKKLGN NCVFAPADVT SEKDVQTALA LAKGKFGRVD VAVNCAGIA VASKTYNLKK GQTHTLEDFQ RVLDVNLMGT FNVIRLVAGE MGQNEPDQGG QRGVIINTAS VAAFEGQVGQ A AYSASKGG IVGMTLPIAR DLAPIGIRVM TIAPGLFGTP LLTSLPEKVC NFLASQVPFP SRLGDPAEYA HLVQAIIENP FL NGEVIRL DGAIRMQP UniProtKB: 3-hydroxyacyl-CoA dehydrogenase type-2 |
-Macromolecule #2: tRNA methyltransferase 10 homolog C
| Macromolecule | Name: tRNA methyltransferase 10 homolog C / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO EC number: Transferases; Transferring one-carbon groups; Methyltransferases |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 24.857826 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: KNFLFLRLWD RNMDIAMGWK GAQAMQFGQP LVFDMAYENY MKRKELQNTV SQLLESEGWN RRNVDPFHIY FCNLKIDGAL HRELVKRYQ EKWDKLLLTS TEKSHVDLFP KDSIIYLTAD SPNVMTTFRH DKVYVIGSFV DKSMQPGTSL AKAKRLNLAT E CLPLDKYL ...String: KNFLFLRLWD RNMDIAMGWK GAQAMQFGQP LVFDMAYENY MKRKELQNTV SQLLESEGWN RRNVDPFHIY FCNLKIDGAL HRELVKRYQ EKWDKLLLTS TEKSHVDLFP KDSIIYLTAD SPNVMTTFRH DKVYVIGSFV DKSMQPGTSL AKAKRLNLAT E CLPLDKYL QWEIGNKNLT LDQMIRILLC LKNNGNWQEA LQFVPKRKHT GFL UniProtKB: tRNA methyltransferase 10 homolog C |
-Macromolecule #3: Mitochondrial Precursor tRNA-Ile(5,4)
| Macromolecule | Name: Mitochondrial Precursor tRNA-Ile(5,4) / type: rna / ID: 3 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 25.118934 KDa |
| Sequence | String: GGGUAAGAAA UAUGUCUGAU AAAAGAGUUA CUUUGAUAGA GUAAAUAAUA GGAGCUUAAA CCCCCUUAUU UCUAGGAC |
-Macromolecule #4: NICOTINAMIDE-ADENINE-DINUCLEOTIDE
| Macromolecule | Name: NICOTINAMIDE-ADENINE-DINUCLEOTIDE / type: ligand / ID: 4 / Number of copies: 4 / Formula: NAD |
|---|---|
| Molecular weight | Theoretical: 663.425 Da |
| Chemical component information |  ChemComp-NAD: |
-Macromolecule #5: S-ADENOSYL-L-HOMOCYSTEINE
| Macromolecule | Name: S-ADENOSYL-L-HOMOCYSTEINE / type: ligand / ID: 5 / Number of copies: 1 / Formula: SAH |
|---|---|
| Molecular weight | Theoretical: 384.411 Da |
| Chemical component information |  ChemComp-SAH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 Component:
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 3 sec. | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 40.1 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: DIFFRACTION / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.9 µm / Nominal magnification: 105000 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: AB INITIO MODEL / Overall B value: 133.7 / Target criteria: Cross-correlation coefficient |
|---|---|
| Output model |  PDB-8cbo: |
 Movie
Movie Controller
Controller



















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)