+Search query
-Structure paper
| Title | Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. |
|---|---|
| Journal, issue, pages | Nat Commun, Vol. 11, Issue 1, Page 2478, Year 2020 |
| Publish date | May 18, 2020 |
 Authors Authors | Chengcheng Guan / Yange Niu / Si-Cong Chen / Yunlu Kang / Jing-Xiang Wu / Koji Nishi / Catherine C Y Chang / Ta-Yuan Chang / Tuoping Luo / Lei Chen /   |
| PubMed Abstract | Sterol O-acyltransferase 1 (SOAT1) is an endoplasmic reticulum (ER) resident, multi-transmembrane enzyme that belongs to the membrane-bound O-acyltransferase (MBOAT) family. It catalyzes the ...Sterol O-acyltransferase 1 (SOAT1) is an endoplasmic reticulum (ER) resident, multi-transmembrane enzyme that belongs to the membrane-bound O-acyltransferase (MBOAT) family. It catalyzes the esterification of cholesterol to generate cholesteryl esters for cholesterol storage. SOAT1 is a target to treat several human diseases. However, its structure and mechanism remain elusive since its discovery. Here, we report the structure of human SOAT1 (hSOAT1) determined by cryo-EM. hSOAT1 is a tetramer consisted of a dimer of dimer. The structure of hSOAT1 dimer at 3.5 Å resolution reveals that a small molecule inhibitor CI-976 binds inside the catalytic chamber and blocks the accessibility of the active site residues H460, N421 and W420. Our results pave the way for future mechanistic study and rational drug design targeting hSOAT1 and other mammalian MBOAT family members. |
 External links External links |  Nat Commun / Nat Commun /  PubMed:32424158 / PubMed:32424158 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 3.5 - 8.25 Å |
| Structure data |  EMDB-0829:  EMDB-0830: |
| Chemicals | 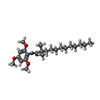 ChemComp-E5L:  ChemComp-CLR: |
| Source |
|
 Keywords Keywords | MEMBRANE PROTEIN / TRANSFERASE / SOAT / ACAT / MBOAT |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers







 homo sapiens (human)
homo sapiens (human)