+検索条件
-Structure paper
| タイトル | Structural insights into branch site proofreading by human spliceosome. |
|---|---|
| ジャーナル・号・ページ | Nat Struct Mol Biol, Vol. 31, Issue 5, Page 835-845, Year 2024 |
| 掲載日 | 2024年1月9日 |
 著者 著者 | Xiaofeng Zhang / Xiechao Zhan / Tong Bian / Fenghua Yang / Pan Li / Yichen Lu / Zhihan Xing / Rongyan Fan / Qiangfeng Cliff Zhang / Yigong Shi /  |
| PubMed 要旨 | Selection of the pre-mRNA branch site (BS) by the U2 small nuclear ribonucleoprotein (snRNP) is crucial to prespliceosome (A complex) assembly. The RNA helicase PRP5 proofreads BS selection but the ...Selection of the pre-mRNA branch site (BS) by the U2 small nuclear ribonucleoprotein (snRNP) is crucial to prespliceosome (A complex) assembly. The RNA helicase PRP5 proofreads BS selection but the underlying mechanism remains unclear. Here we report the atomic structures of two sequential complexes leading to prespliceosome assembly: human 17S U2 snRNP and a cross-exon pre-A complex. PRP5 is anchored on 17S U2 snRNP mainly through occupation of the RNA path of SF3B1 by an acidic loop of PRP5; the helicase domain of PRP5 associates with U2 snRNA; the BS-interacting stem-loop (BSL) of U2 snRNA is shielded by TAT-SF1, unable to engage the BS. In the pre-A complex, an initial U2-BS duplex is formed; the translocated helicase domain of PRP5 stays with U2 snRNA and the acidic loop still occupies the RNA path. The pre-A conformation is specifically stabilized by the splicing factors SF1, DNAJC8 and SF3A2. Cancer-derived mutations in SF3B1 damage its association with PRP5, compromising BS proofreading. Together, these findings reveal key insights into prespliceosome assembly and BS selection or proofreading by PRP5. |
 リンク リンク |  Nat Struct Mol Biol / Nat Struct Mol Biol /  PubMed:38196034 PubMed:38196034 |
| 手法 | EM (単粒子) |
| 解像度 | 2.5 - 3.0 Å |
| 構造データ | EMDB-31334: The cryo-EM map of the human 17S U2 snRNP core region EMDB-32074, PDB-7vpx: |
| 化合物 | 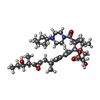 ChemComp-9B0:  ChemComp-ZN: 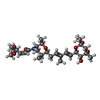 ChemComp-SJT: |
| 由来 |
|
 キーワード キーワード | SPLICING / U2 snRNP / PRP5 / SF3B1 / human spliceosome / pre-A complex / A complex / U1 snRNP |
 ムービー
ムービー コントローラー
コントローラー 構造ビューア
構造ビューア 万見文献について
万見文献について







 homo sapiens (ヒト)
homo sapiens (ヒト) unidentified adenovirus (ウイルス)
unidentified adenovirus (ウイルス)