+検索条件
-Structure paper
| タイトル | eIF2B conformation and assembly state regulate the integrated stress response. |
|---|---|
| ジャーナル・号・ページ | Elife, Vol. 10, Year 2021 |
| 掲載日 | 2021年3月10日 |
 著者 著者 | Michael Schoof / Morgane Boone / Lan Wang / Rosalie Lawrence / Adam Frost / Peter Walter /  |
| PubMed 要旨 | The integrated stress response (ISR) is activated by phosphorylation of the translation initiation factor eIF2 in response to various stress conditions. Phosphorylated eIF2 (eIF2-P) inhibits eIF2's ...The integrated stress response (ISR) is activated by phosphorylation of the translation initiation factor eIF2 in response to various stress conditions. Phosphorylated eIF2 (eIF2-P) inhibits eIF2's nucleotide exchange factor eIF2B, a twofold symmetric heterodecamer assembled from subcomplexes. Here, we monitor and manipulate eIF2B assembly in vitro and in vivo. In the absence of eIF2B's α-subunit, the ISR is induced because unassembled eIF2B tetramer subcomplexes accumulate in cells. Upon addition of the small-molecule ISR inhibitor ISRIB, eIF2B tetramers assemble into active octamers. Surprisingly, ISRIB inhibits the ISR even in the context of fully assembled eIF2B decamers, revealing allosteric communication between the physically distant eIF2, eIF2-P, and ISRIB binding sites. Cryo-electron microscopy structures suggest a rocking motion in eIF2B that couples these binding sites. eIF2-P binding converts eIF2B decamers into 'conjoined tetramers' with diminished substrate binding and enzymatic activity. Canonical eIF2-P-driven ISR activation thus arises due to this change in eIF2B's conformational state. |
 リンク リンク |  Elife / Elife /  PubMed:33688831 / PubMed:33688831 /  PubMed Central PubMed Central |
| 手法 | EM (単粒子) |
| 解像度 | 2.8 - 3 Å |
| 構造データ | EMDB-23209, PDB-7l70:  PDB-7l7g: |
| 化合物 | 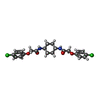 ChemComp-C7B: |
| 由来 |
|
 キーワード キーワード | TRANSLATION / integrated stress response / Translation; Integrated stress response |
 ムービー
ムービー コントローラー
コントローラー 構造ビューア
構造ビューア 万見文献について
万見文献について





 homo sapiens (ヒト)
homo sapiens (ヒト)