+Search query
-Structure paper
| Title | Structural basis for substrate binding and selection by human mitochondrial RNA polymerase. |
|---|---|
| Journal, issue, pages | Nat Commun, Vol. 15, Issue 1, Page 7134, Year 2024 |
| Publish date | Aug 20, 2024 |
 Authors Authors | Karl Herbine / Ashok R Nayak / Dmitry Temiakov /  |
| PubMed Abstract | The mechanism by which RNAP selects cognate substrates and discriminates between deoxy and ribonucleotides is of fundamental importance to the fidelity of transcription. Here, we present cryo-EM ...The mechanism by which RNAP selects cognate substrates and discriminates between deoxy and ribonucleotides is of fundamental importance to the fidelity of transcription. Here, we present cryo-EM structures of human mitochondrial transcription elongation complexes that reveal substrate ATP bound in Entry and Insertion Sites. In the Entry Site, the substrate binds along the O helix of the fingers domain of mtRNAP but does not interact with the templating DNA base. Interactions between RNAP and the triphosphate moiety of the NTP in the Entry Site ensure discrimination against nucleosides and their diphosphate and monophosphate derivatives but not against non-cognate rNTPs and dNTPs. Closing of the fingers domain over the catalytic site results in delivery of both the templating DNA base and the substrate into the Insertion Site and recruitment of the catalytic magnesium ions. The cryo-EM data also reveal a conformation adopted by mtRNAP to reject a non-cognate substrate from its active site. Our findings establish a structural basis for substrate binding and suggest a unified mechanism of NTP selection for single-subunit RNAPs. |
 External links External links |  Nat Commun / Nat Commun /  PubMed:39164235 / PubMed:39164235 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 2.54 - 2.9 Å |
| Structure data | EMDB-42027, PDB-8u8u: EMDB-42028, PDB-8u8v: EMDB-44448, PDB-9bdc: EMDB-44449, PDB-9bdd: |
| Chemicals | 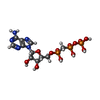 ChemComp-APC:  ChemComp-MG: |
| Source |
|
 Keywords Keywords | TRANSCRIPTION/DNA/RNA / Mitochondrial RNA Polymerase / Nucleotide Selection / Nucleotide Discrimination / TRANSCRIPTION / Protein-RNA-DNA Complex / POLRMT / TRANSCRIPTION-DNA-RNA complex / TEFM |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers











 homo sapiens (human)
homo sapiens (human)