+Search query
-Structure paper
| Title | Helical ultrastructure of the metalloprotease meprin α in complex with a small molecule inhibitor. |
|---|---|
| Journal, issue, pages | Nat Commun, Vol. 13, Issue 1, Page 6178, Year 2022 |
| Publish date | Oct 19, 2022 |
 Authors Authors | Charles Bayly-Jones / Christopher J Lupton / Claudia Fritz / Hariprasad Venugopal / Daniel Ramsbeck / Michael Wermann / Christian Jäger / Alex de Marco / Stephan Schilling / Dagmar Schlenzig / James C Whisstock /   |
| PubMed Abstract | The zinc-dependent metalloprotease meprin α is predominantly expressed in the brush border membrane of proximal tubules in the kidney and enterocytes in the small intestine and colon. In normal ...The zinc-dependent metalloprotease meprin α is predominantly expressed in the brush border membrane of proximal tubules in the kidney and enterocytes in the small intestine and colon. In normal tissue homeostasis meprin α performs key roles in inflammation, immunity, and extracellular matrix remodelling. Dysregulated meprin α is associated with acute kidney injury, sepsis, urinary tract infection, metastatic colorectal carcinoma, and inflammatory bowel disease. Accordingly, meprin α is the target of drug discovery programs. In contrast to meprin β, meprin α is secreted into the extracellular space, whereupon it oligomerises to form giant assemblies and is the largest extracellular protease identified to date (~6 MDa). Here, using cryo-electron microscopy, we determine the high-resolution structure of the zymogen and mature form of meprin α, as well as the structure of the active form in complex with a prototype small molecule inhibitor and human fetuin-B. Our data reveal that meprin α forms a giant, flexible, left-handed helical assembly of roughly 22 nm in diameter. We find that oligomerisation improves proteolytic and thermal stability but does not impact substrate specificity or enzymatic activity. Furthermore, structural comparison with meprin β reveal unique features of the active site of meprin α, and helical assembly more broadly. |
 External links External links |  Nat Commun / Nat Commun /  PubMed:36261433 / PubMed:36261433 /  PubMed Central PubMed Central |
| Methods | EM (single particle) / EM (subtomogram averaging) |
| Resolution | 2.4 - 12.7 Å |
| Structure data | EMDB-26419: Human pro-meprin alpha (zymogen state)[subparticle localised reconstruction] EMDB-26420: Human pro-meprin alpha (zymogen state)[Focused classification and refinement]  EMDB-26421: Meprin alpha helix (activated) - full C1 consensus reconstruction EMDB-26422: Human meprin alpha (active state)[subparticle localised reconstruction] EMDB-26423, PDB-7uaf:  EMDB-26424: Meprin alpha helix in complex with fetuin-B [consensus C1 reconstruction] EMDB-26426: Meprin alpha helix in complex with fetuin-B [subparticle localised reconstruction, masked focused refinement]  EMDB-27689: Sub-tomogram average of pro-meprin alpha supercoiled filament |
| Chemicals |  ChemComp-NAG:  ChemComp-CA:  ChemComp-ZN: 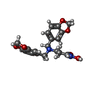 ChemComp-M6X: |
| Source |
|
 Keywords Keywords | ONCOPROTEIN / Metalloprotease / complex / helical / extracellular |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers













 homo sapiens (human)
homo sapiens (human)