+Search query
-Structure paper
| Title | Cryo-EM reveals an extrahelical allosteric binding site at the M mAChR. |
|---|---|
| Journal, issue, pages | Nat Commun, Vol. 16, Issue 1, Page 7046, Year 2025 |
| Publish date | Jul 31, 2025 |
 Authors Authors | Wessel A C Burger / Jesse I Mobbs / Bhavika Rana / Jinan Wang / Keya Joshi / Patrick R Gentry / Mahmuda Yeasmin / Hariprasad Venugopal / Aaron M Bender / Craig W Lindsley / Yinglong Miao / Arthur Christopoulos / Celine Valant / David M Thal /   |
| PubMed Abstract | The M muscarinic acetylcholine receptor (M mAChR) represents a promising therapeutic target for neurological disorders. However, the high conservation of its orthosteric binding site poses ...The M muscarinic acetylcholine receptor (M mAChR) represents a promising therapeutic target for neurological disorders. However, the high conservation of its orthosteric binding site poses significant challenges for drug development. While selective positive allosteric modulators (PAMs) offer a potential solution, a structural understanding of the M mAChR and its allosteric binding sites remains limited. Here, we present a 2.8 Å cryo-electron microscopy structure of the M mAChR complexed with heterotrimeric G protein and the agonist iperoxo, completing the active-state structural characterization of the mAChR family. To identify the binding site of M-selective PAMs, we implement an integrated approach combining mutagenesis, pharmacological assays, structural biology, and molecular dynamics simulations. Our mutagenesis studies reveal that selective M PAMs bind outside previously characterized M mAChR allosteric sites. Subsequently, we obtain a 2.1 Å structure of M mAChR co-bound with acetylcholine and the selective PAM VU6007678, revealing an allosteric pocket at the extrahelical interface between transmembrane domains 3 and 4 that is confirmed through mutagenesis and simulations. These findings demonstrate the diverse mechanisms of allosteric regulation in mAChRs and highlight the value of integrating pharmacological and structural approaches to identify allosteric binding sites. |
 External links External links |  Nat Commun / Nat Commun /  PubMed:40745154 / PubMed:40745154 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 2.06 - 2.79 Å |
| Structure data | EMDB-48110, PDB-9ejz: EMDB-48111, PDB-9ek0: |
| Chemicals |  PDB-1bks: 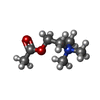 ChemComp-ACH:  ChemComp-HOH: 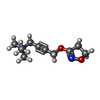 ChemComp-IXO: |
| Source |
|
 Keywords Keywords | MEMBRANE PROTEIN / G protein-coupled receptor / acetylcholine binding / seven transmembrane protein |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers







 homo sapiens (human)
homo sapiens (human)
