+検索条件
-Structure paper
| タイトル | Sterols in an intramolecular channel of Smoothened mediate Hedgehog signaling. |
|---|---|
| ジャーナル・号・ページ | Nat Chem Biol, Vol. 16, Issue 12, Page 1368-1375, Year 2020 |
| 掲載日 | 2020年9月14日 |
 著者 著者 | Xiaofeng Qi / Lucas Friedberg / Ryan De Bose-Boyd / Tao Long / Xiaochun Li /  |
| PubMed 要旨 | Smoothened (SMO), a class Frizzled G protein-coupled receptor (class F GPCR), transduces the Hedgehog signal across the cell membrane. Sterols can bind to its extracellular cysteine-rich domain ...Smoothened (SMO), a class Frizzled G protein-coupled receptor (class F GPCR), transduces the Hedgehog signal across the cell membrane. Sterols can bind to its extracellular cysteine-rich domain (CRD) and to several sites in the seven transmembrane helices (7-TMs) of SMO. However, the mechanism by which sterols regulate SMO via multiple sites is unknown. Here we determined the structures of SMO-G complexes bound to the synthetic SMO agonist (SAG) and to 24(S),25-epoxycholesterol (24(S),25-EC). A novel sterol-binding site in the extracellular extension of TM6 was revealed to connect other sites in 7-TMs and CRD, forming an intramolecular sterol channel from the middle side of 7-TMs to CRD. Additional structures of two gain-of-function variants, SMO and SMO, showed that blocking the channel at its midpoints allows sterols to occupy the binding sites in 7-TMs, thereby activating SMO. These data indicate that sterol transport through the core of SMO is a major regulator of SMO-mediated signaling. |
 リンク リンク |  Nat Chem Biol / Nat Chem Biol /  PubMed:32929279 / PubMed:32929279 /  PubMed Central PubMed Central |
| 手法 | EM (単粒子) |
| 解像度 | 3.14 - 3.96 Å |
| 構造データ | EMDB-22117, PDB-6xbj: EMDB-22118, PDB-6xbk: EMDB-22119, PDB-6xbl: EMDB-22120, PDB-6xbm: |
| 化合物 |  ChemComp-CLR: 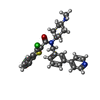 ChemComp-V0S: 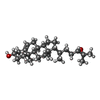 ChemComp-CO1: |
| 由来 |
|
 キーワード キーワード | MEMBRANE PROTEIN / GPCR |
 ムービー
ムービー コントローラー
コントローラー 構造ビューア
構造ビューア 万見文献について
万見文献について











 homo sapiens (ヒト)
homo sapiens (ヒト)
