+検索条件
-Structure paper
| タイトル | Functional reconstitution of human eukaryotic translation initiation factor 3 (eIF3). |
|---|---|
| ジャーナル・号・ページ | Proc Natl Acad Sci U S A, Vol. 108, Issue 51, Page 20473-20478, Year 2011 |
| 掲載日 | 2011年12月20日 |
 著者 著者 | Chaomin Sun / Aleksandar Todorovic / Jordi Querol-Audí / Yun Bai / Nancy Villa / Monica Snyder / John Ashchyan / Christopher S Lewis / Abbey Hartland / Scott Gradia / Christopher S Fraser / Jennifer A Doudna / Eva Nogales / Jamie H D Cate /  |
| PubMed 要旨 | Protein fate in higher eukaryotes is controlled by three complexes that share conserved architectural elements: the proteasome, COP9 signalosome, and eukaryotic translation initiation factor 3 (eIF3). ...Protein fate in higher eukaryotes is controlled by three complexes that share conserved architectural elements: the proteasome, COP9 signalosome, and eukaryotic translation initiation factor 3 (eIF3). Here we reconstitute the 13-subunit human eIF3 in Escherichia coli, revealing its structural core to be the eight subunits with conserved orthologues in the proteasome lid complex and COP9 signalosome. This structural core in eIF3 binds to the small (40S) ribosomal subunit, to translation initiation factors involved in mRNA cap-dependent initiation, and to the hepatitis C viral (HCV) internal ribosome entry site (IRES) RNA. Addition of the remaining eIF3 subunits enables reconstituted eIF3 to assemble intact initiation complexes with the HCV IRES. Negative-stain EM reconstructions of reconstituted eIF3 further reveal how the approximately 400 kDa molecular mass structural core organizes the highly flexible 800 kDa molecular mass eIF3 complex, and mediates translation initiation. |
 リンク リンク |  Proc Natl Acad Sci U S A / Proc Natl Acad Sci U S A /  PubMed:22135459 / PubMed:22135459 /  PubMed Central PubMed Central |
| 手法 | EM (単粒子) |
| 解像度 | 23.0 - 29.3 Å |
| 構造データ | 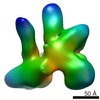 EMDB-1975:  EMDB-1976: |
| 由来 |
|
 ムービー
ムービー コントローラー
コントローラー 構造ビューア
構造ビューア 万見文献について
万見文献について



 Homo sapiens (ヒト)
Homo sapiens (ヒト)