[English] 日本語
 Yorodumi
Yorodumi- EMDB-9263: Single-Molecule 3D Image of Double Complexes of DNA-Nanogold Conj... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-9263 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Single-Molecule 3D Image of Double Complexes of DNA-Nanogold Conjugates (No. 02) | |||||||||
 Map data Map data | Double Complexes of DNA-Nanogold Conjugates (No. 02) | |||||||||
 Sample Sample |
| |||||||||
| Biological species | synthetic construct (others) | |||||||||
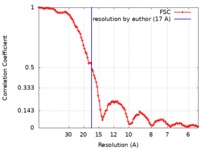
| Method | electron tomography / negative staining / Resolution: 17.0 Å | |||||||||
 Authors Authors | Wu H / Zhai X / Lei D / Liu J / Yu Y / Bie R / Ren G | |||||||||
 Citation Citation |  Journal: Sci Rep / Year: 2018 Journal: Sci Rep / Year: 2018Title: An Algorithm for Enhancing the Image Contrast of Electron Tomography. Authors: Hao Wu / Xiaobo Zhai / Dongsheng Lei / Jianfang Liu / Yadong Yu / Rongfang Bie / Gang Ren /   Abstract: Three-dimensional (3D) reconstruction of a single protein molecule is essential for understanding the relationship between the structural dynamics and functions of the protein. Electron tomography ...Three-dimensional (3D) reconstruction of a single protein molecule is essential for understanding the relationship between the structural dynamics and functions of the protein. Electron tomography (ET) provides a tool for imaging an individual particle of protein from a series of tilted angles. Individual-particle electron tomography (IPET) provides an approach for reconstructing a 3D density map from a single targeted protein particle (without averaging from different particles of this type of protein), in which the target particle was imaged from a series of tilting angles. However, owing to radiation damage limitations, low-dose images (high noise, and low image contrast) are often challenging to be aligned for 3D reconstruction at intermediate resolution (1-3 nm). Here, we propose a computational method to enhance the image contrast, without increasing any experimental dose, for IPET 3D reconstruction. Using an edge-preserving smoothing-based multi-scale image decomposition algorithm, this method can detect the object against a high-noise background and enhance the object image contrast without increasing the noise level or significantly decreasing the image resolution. The method was validated by using both negative staining (NS) ET and cryo-ET images. The successful 3D reconstruction of a small molecule (<100 kDa) indicated that this method can be used as a supporting tool to current ET 3D reconstruction methods for studying protein dynamics via structure determination from each individual particle of the same type of protein. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_9263.map.gz emd_9263.map.gz | 93.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-9263-v30.xml emd-9263-v30.xml emd-9263.xml emd-9263.xml | 11.2 KB 11.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_9263_fsc.xml emd_9263_fsc.xml | 10.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_9263.png emd_9263.png | 37.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-9263 http://ftp.pdbj.org/pub/emdb/structures/EMD-9263 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9263 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9263 | HTTPS FTP |
-Validation report
| Summary document |  emd_9263_validation.pdf.gz emd_9263_validation.pdf.gz | 79.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_9263_full_validation.pdf.gz emd_9263_full_validation.pdf.gz | 78.2 KB | Display | |
| Data in XML |  emd_9263_validation.xml.gz emd_9263_validation.xml.gz | 494 B | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9263 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9263 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9263 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9263 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_9263.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_9263.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Double Complexes of DNA-Nanogold Conjugates (No. 02) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.82 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
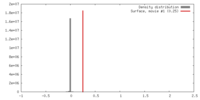
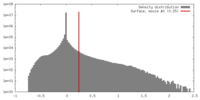
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Double complexes of 84-base pair double-stranded DNA bound with t...
| Entire | Name: Double complexes of 84-base pair double-stranded DNA bound with two 5-nm nanogolds |
|---|---|
| Components |
|
-Supramolecule #1: Double complexes of 84-base pair double-stranded DNA bound with t...
| Supramolecule | Name: Double complexes of 84-base pair double-stranded DNA bound with two 5-nm nanogolds type: complex / ID: 1 / Parent: 0 Details: 5-nm nanogold particles were stabilized via exchanging with bis-(p-sulfonatophenyl) phenylphosphine (BSPP). DNA sequences modified with a 5 thiol moiety were PAGE purified. DNA thiolated at ...Details: 5-nm nanogold particles were stabilized via exchanging with bis-(p-sulfonatophenyl) phenylphosphine (BSPP). DNA sequences modified with a 5 thiol moiety were PAGE purified. DNA thiolated at the 5 end was re-suspended in buffer (10mM Tris pH 8, 0.5mM EDTA). Nanogold particles and DNA were combined at a stoichiometric ratio of 1:2 in the presence of a reducing agent. Monoconjugates formed were separated by anion exchange HPLC, and the fractions concentrated by an Amicon Ultra spin filter, MW 100,000 (EMD Millipore Corp, Billerica, MA). Twenty microliters of nanogold monoconjugates, each containing complementary strands of DNA, were combined stoichiometrically as determined by absorption at 520 nm and allowed to react overnight at room temperature. The dimers were purified from unreacted monoconjugates by agarose gel electrophoresis. |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
-Experimental details
-Structure determination
| Method | negative staining |
|---|---|
 Processing Processing | electron tomography |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.02 mg/mL |
|---|---|
| Buffer | pH: 7 Details: 1X Dulbeccos phosphate-buffered saline, 2.7 mM KCl, 1.46 mM KH2PO4, 136.9 mM NaCl, and 8.1 mM Na2HPO4 |
| Staining | Type: NEGATIVE / Material: Uranyl Formate Details: The grid was stained with 1% (w/v) uranyl formate by using optimized negative-staining (OpNS) protocol. |
| Grid | Model: Homemade / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR |
| Details | 5-nm nanogold particles were stabilized via exchanging with bis-(p-sulfonatophenyl) phenylphosphine (BSPP). DNA sequences modified with a 5 thiol moiety were PAGE purified. DNA thiolated at the 5 end was re-suspended in buffer (10mM Tris pH 8, 0.5mM EDTA). Nanogold particles and DNA were combined at a stoichiometric ratio of 1:2 in the presence of a reducing agent. Monoconjugates formed were separated by anion exchange HPLC, and the fractions concentrated by an Amicon Ultra spin filter, MW 100,000 (EMD Millipore Corp, Billerica, MA). Twenty microliters of nanogold monoconjugates, each containing complementary strands of DNA, were combined stoichiometrically as determined by absorption at 520 nm and allowed to react overnight at room temperature. The dimers were purified from unreacted monoconjugates by agarose gel electrophoresis. |
| Sectioning | Other: NO SECTIONING |
- Electron microscopy
Electron microscopy
| Microscope | ZEISS LIBRA120PLUS |
|---|---|
| Specialist optics | Energy filter - Name: In-column Omega Filter / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN ULTRASCAN 4000 (4k x 4k) / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Average electron dose: 56.39 e/Å2 |
| Electron beam | Acceleration voltage: 120 kV / Electron source: LAB6 |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.2 mm / Nominal magnification: 125000 |
 Movie
Movie Controller
Controller


 UCSF Chimera
UCSF Chimera







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)