[English] 日本語
 Yorodumi
Yorodumi- EMDB-51241: Structure of Chd1 bound to a hexasome-nucleosome complex with a d... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of Chd1 bound to a hexasome-nucleosome complex with a dyad-to-dyad distance of 103 bp. | |||||||||
 Map data Map data | Composite map of hexasome-nucleosome complex SHN103 bound by Chd1 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | chromatin / remodeling / transcription / nucleosome / DNA BINDING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationnucleolar chromatin / regulation of transcriptional start site selection at RNA polymerase II promoter / negative regulation of DNA-templated DNA replication / regulation of chromatin organization / rDNA binding / SLIK (SAGA-like) complex / DNA double-strand break processing / nucleosome organization / ATP-dependent chromatin remodeler activity / SAGA complex ...nucleolar chromatin / regulation of transcriptional start site selection at RNA polymerase II promoter / negative regulation of DNA-templated DNA replication / regulation of chromatin organization / rDNA binding / SLIK (SAGA-like) complex / DNA double-strand break processing / nucleosome organization / ATP-dependent chromatin remodeler activity / SAGA complex / sister chromatid cohesion / termination of RNA polymerase II transcription / termination of RNA polymerase I transcription / ATP-dependent activity, acting on DNA / : / helicase activity / transcription elongation by RNA polymerase II / double-strand break repair via homologous recombination / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / chromatin DNA binding / structural constituent of chromatin / nucleosome / site of double-strand break / histone binding / transcription cis-regulatory region binding / chromatin remodeling / protein heterodimerization activity / chromatin binding / regulation of transcription by RNA polymerase II / chromatin / ATP hydrolysis activity / mitochondrion / DNA binding / nucleoplasm / ATP binding / nucleus Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.0 Å | |||||||||
 Authors Authors | Engeholm M / Roske JJ / Oberbeckmann E / Dienemann C / Lidschreiber M / Cramer P / Farnung L | |||||||||
| Funding support |  Germany, European Union, 2 items Germany, European Union, 2 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2024 Journal: Mol Cell / Year: 2024Title: Resolution of transcription-induced hexasome-nucleosome complexes by Chd1 and FACT. Authors: Maik Engeholm / Johann J Roske / Elisa Oberbeckmann / Christian Dienemann / Michael Lidschreiber / Patrick Cramer / Lucas Farnung /    Abstract: To maintain the nucleosome organization of transcribed genes, ATP-dependent chromatin remodelers collaborate with histone chaperones. Here, we show that at the 5' ends of yeast genes, RNA polymerase ...To maintain the nucleosome organization of transcribed genes, ATP-dependent chromatin remodelers collaborate with histone chaperones. Here, we show that at the 5' ends of yeast genes, RNA polymerase II (RNAPII) generates hexasomes that occur directly adjacent to nucleosomes. The resulting hexasome-nucleosome complexes are then resolved by Chd1. We present two cryoelectron microscopy (cryo-EM) structures of Chd1 bound to a hexasome-nucleosome complex before and after restoration of the missing inner H2A/H2B dimer by FACT. Chd1 uniquely interacts with the complex, positioning its ATPase domain to shift the hexasome away from the nucleosome. In the absence of the inner H2A/H2B dimer, its DNA-binding domain (DBD) packs against the ATPase domain, suggesting an inhibited state. Restoration of the dimer by FACT triggers a rearrangement that displaces the DBD and stimulates Chd1 remodeling. Our results demonstrate how chromatin remodelers interact with a complex nucleosome assembly and suggest how Chd1 and FACT jointly support transcription by RNAPII. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_51241.map.gz emd_51241.map.gz | 263.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-51241-v30.xml emd-51241-v30.xml emd-51241.xml emd-51241.xml | 20.9 KB 20.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_51241.png emd_51241.png | 106.9 KB | ||
| Filedesc metadata |  emd-51241.cif.gz emd-51241.cif.gz | 7.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-51241 http://ftp.pdbj.org/pub/emdb/structures/EMD-51241 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-51241 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-51241 | HTTPS FTP |
-Validation report
| Summary document |  emd_51241_validation.pdf.gz emd_51241_validation.pdf.gz | 453.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_51241_full_validation.pdf.gz emd_51241_full_validation.pdf.gz | 453.3 KB | Display | |
| Data in XML |  emd_51241_validation.xml.gz emd_51241_validation.xml.gz | 7.2 KB | Display | |
| Data in CIF |  emd_51241_validation.cif.gz emd_51241_validation.cif.gz | 8.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-51241 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-51241 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-51241 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-51241 | HTTPS FTP |
-Related structure data
| Related structure data |  9gd1MC  9gd0C  9gd2C  9gd3C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_51241.map.gz / Format: CCP4 / Size: 282.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_51241.map.gz / Format: CCP4 / Size: 282.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Composite map of hexasome-nucleosome complex SHN103 bound by Chd1 | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.834 Å | ||||||||||||||||||||||||||||||||||||
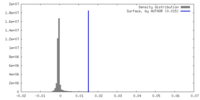
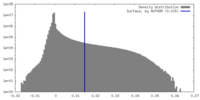
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
+Entire : Chd1 bound to a hexasome-nucleosome complex with a dyad-to-dyad d...
+Supramolecule #1: Chd1 bound to a hexasome-nucleosome complex with a dyad-to-dyad d...
+Macromolecule #1: Histone H3.2
+Macromolecule #2: Histone H4
+Macromolecule #3: Histone H2A type 1
+Macromolecule #4: Histone H2B 1.1
+Macromolecule #7: Chromo domain-containing protein 1
+Macromolecule #5: DNA (261-MER)
+Macromolecule #6: DNA (261-MER)
+Macromolecule #8: ADENOSINE-5'-DIPHOSPHATE
+Macromolecule #9: BERYLLIUM TRIFLUORIDE ION
+Macromolecule #10: MAGNESIUM ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 39.8 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 4.0 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 26819 |
| Initial angle assignment | Type: OTHER |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)



















 Trichoplusia ni (cabbage looper)
Trichoplusia ni (cabbage looper)

