[English] 日本語
 Yorodumi
Yorodumi- EMDB-43700: Cryo-EM map of LKB1-STRADalpha-MO25alpha from TFS Glacios with Ga... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM map of LKB1-STRADalpha-MO25alpha from TFS Glacios with Gatan Alpine detector at 120 keV | |||||||||
 Map data Map data | Cryo-EM map of LKB1-STRADalpha-MO25alpha from TFS Glacios with Gatan Alpine detector at 120 keV | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | serine/threonine kinase / pseudokinase / complex / TRANSFERASE | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
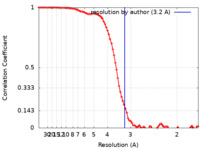
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Chan LM / Courteau BJ / Verba KA | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: J Struct Biol / Year: 2024 Journal: J Struct Biol / Year: 2024Title: High-resolution single-particle imaging at 100-200 keV with the Gatan Alpine direct electron detector. Authors: Lieza M Chan / Brandon J Courteau / Allison Maker / Mengyu Wu / Benjamin Basanta / Hev Mehmood / David Bulkley / David Joyce / Brian C Lee / Stephen Mick / Cory Czarnik / Sahil Gulati / ...Authors: Lieza M Chan / Brandon J Courteau / Allison Maker / Mengyu Wu / Benjamin Basanta / Hev Mehmood / David Bulkley / David Joyce / Brian C Lee / Stephen Mick / Cory Czarnik / Sahil Gulati / Gabriel C Lander / Kliment A Verba /  Abstract: Developments in direct electron detector technology have played a pivotal role in enabling high-resolution structural studies by cryo-EM at 200 and 300 keV. Yet, theory and recent experiments ...Developments in direct electron detector technology have played a pivotal role in enabling high-resolution structural studies by cryo-EM at 200 and 300 keV. Yet, theory and recent experiments indicate advantages to imaging at 100 keV, energies for which the current detectors have not been optimized. In this study, we evaluated the Gatan Alpine detector, designed for operation at 100 and 200 keV. Compared to the Gatan K3, Alpine demonstrated a significant DQE improvement at these energies, specifically a ∼ 4-fold improvement at Nyquist at 100 keV. In single-particle cryo-EM experiments, Alpine datasets yielded better than 2 Å resolution reconstructions of apoferritin at 120 and 200 keV on a ThermoFisher Scientific (TFS) Glacios microscope fitted with a non-standard SP-Twin lens. We also achieved a ∼ 3.2 Å resolution reconstruction of a 115 kDa asymmetric protein complex, proving Alpine's effectiveness with complex biological samples. In-depth analysis revealed that Alpine reconstructions are comparable to K3 reconstructions at 200 keV, and remarkably, reconstruction from Alpine at 120 keV on a TFS Glacios surpassed all but the 300 keV data from a TFS Titan Krios with GIF/K3. Additionally, we show Alpine's capability for high-resolution data acquisition and screening on lower-end systems by obtaining ∼ 3 Å resolution reconstructions of apoferritin and aldolase at 100 keV and detailed 2D averages of a 55 kDa sample using a side-entry cryo holder. Overall, we show that Gatan Alpine performs well with the standard 200 keV imaging systems and may potentially capture the benefits of lower accelerating voltages, bringing smaller sized particles within the scope of cryo-EM. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_43700.map.gz emd_43700.map.gz | 59.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-43700-v30.xml emd-43700-v30.xml emd-43700.xml emd-43700.xml | 17 KB 17 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_43700_fsc.xml emd_43700_fsc.xml | 8.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_43700.png emd_43700.png | 80.6 KB | ||
| Filedesc metadata |  emd-43700.cif.gz emd-43700.cif.gz | 4.3 KB | ||
| Others |  emd_43700_additional_1.map.gz emd_43700_additional_1.map.gz emd_43700_half_map_1.map.gz emd_43700_half_map_1.map.gz emd_43700_half_map_2.map.gz emd_43700_half_map_2.map.gz | 31.7 MB 59.5 MB 59.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-43700 http://ftp.pdbj.org/pub/emdb/structures/EMD-43700 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43700 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43700 | HTTPS FTP |
-Validation report
| Summary document |  emd_43700_validation.pdf.gz emd_43700_validation.pdf.gz | 877.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_43700_full_validation.pdf.gz emd_43700_full_validation.pdf.gz | 877.1 KB | Display | |
| Data in XML |  emd_43700_validation.xml.gz emd_43700_validation.xml.gz | 16.5 KB | Display | |
| Data in CIF |  emd_43700_validation.cif.gz emd_43700_validation.cif.gz | 21.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43700 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43700 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43700 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43700 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_43700.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_43700.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM map of LKB1-STRADalpha-MO25alpha from TFS Glacios with Gatan Alpine detector at 120 keV | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.85 Å | ||||||||||||||||||||||||||||||||||||
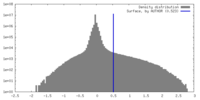
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Additional Map
| File | emd_43700_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Additional Map | ||||||||||||
| Projections & Slices |
| ||||||||||||
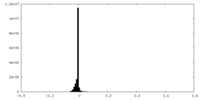
| Density Histograms |
-Half map: Half Map B
| File | emd_43700_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
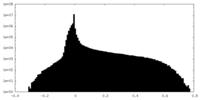
| Density Histograms |
-Half map: Half Map A
| File | emd_43700_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
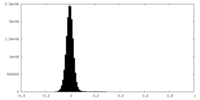
| Density Histograms |
- Sample components
Sample components
-Entire : Heterotrimeric complex of serine/threonine kinase LKB1 with psued...
| Entire | Name: Heterotrimeric complex of serine/threonine kinase LKB1 with psuedokinase STRADa and scaffolding MO25a |
|---|---|
| Components |
|
-Supramolecule #1: Heterotrimeric complex of serine/threonine kinase LKB1 with psued...
| Supramolecule | Name: Heterotrimeric complex of serine/threonine kinase LKB1 with psuedokinase STRADa and scaffolding MO25a type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 140.5 KDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.6 Component:
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Support film - Material: CARBON / Support film - topology: HOLEY | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK III |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Image recording | Film or detector model: GATAN ALPINE (2.3k x 3.2k) / Number grids imaged: 1 / Number real images: 3635 / Average electron dose: 58.9 e/Å2 |
| Electron beam | Acceleration voltage: 120 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 1.7 mm / Nominal defocus max: 1.2 µm / Nominal defocus min: 0.7000000000000001 µm / Nominal magnification: 43000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)