[English] 日本語
 Yorodumi
Yorodumi- EMDB-42288: Cryo-EM map of human clamp-clamp loader ATAD5-RFC-two PCNAs compl... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM map of human clamp-clamp loader ATAD5-RFC-two PCNAs complex in intermediate state 3 | ||||||||||||
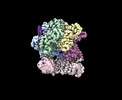
 Map data Map data | Final sharpened Cryo-EM map | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | AAA ATPase / Clamp unloader / MOTOR PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of cell cycle G2/M phase transition / DNA clamp unloader activity / positive regulation of deoxyribonuclease activity / dinucleotide insertion or deletion binding / PCNA-p21 complex / response to organophosphorus / Ctf18 RFC-like complex / mitotic telomere maintenance via semi-conservative replication / nuclear DNA replication / Rad17 RFC-like complex ...positive regulation of cell cycle G2/M phase transition / DNA clamp unloader activity / positive regulation of deoxyribonuclease activity / dinucleotide insertion or deletion binding / PCNA-p21 complex / response to organophosphorus / Ctf18 RFC-like complex / mitotic telomere maintenance via semi-conservative replication / nuclear DNA replication / Rad17 RFC-like complex / DNA replication factor C complex / Elg1 RFC-like complex / purine-specific mismatch base pair DNA N-glycosylase activity / positive regulation of isotype switching to IgG isotypes / positive regulation of DNA-directed DNA polymerase activity / nuclear lamina / MutLalpha complex binding / Polymerase switching / Telomere C-strand (Lagging Strand) Synthesis / Processive synthesis on the lagging strand / DNA clamp loader activity / PCNA complex / Removal of the Flap Intermediate / Processive synthesis on the C-strand of the telomere / isotype switching / Mismatch repair (MMR) directed by MSH2:MSH3 (MutSbeta) / Polymerase switching on the C-strand of the telomere / Mismatch repair (MMR) directed by MSH2:MSH6 (MutSalpha) / Transcription of E2F targets under negative control by DREAM complex / Removal of the Flap Intermediate from the C-strand / replisome / DNA duplex unwinding / regulation of mitotic cell cycle phase transition / response to L-glutamate / HDR through Single Strand Annealing (SSA) / Impaired BRCA2 binding to RAD51 / DNA strand elongation involved in DNA replication / histone acetyltransferase binding / DNA synthesis involved in DNA repair / DNA polymerase processivity factor activity / G1/S-Specific Transcription / negative regulation of intrinsic apoptotic signaling pathway in response to DNA damage by p53 class mediator / leading strand elongation / response to dexamethasone / replication fork processing / nuclear replication fork / Presynaptic phase of homologous DNA pairing and strand exchange / SUMOylation of DNA replication proteins / estrous cycle / PCNA-Dependent Long Patch Base Excision Repair / cyclin-dependent protein kinase holoenzyme complex / intrinsic apoptotic signaling pathway in response to DNA damage by p53 class mediator / signal transduction in response to DNA damage / ATP-dependent activity, acting on DNA / mismatch repair / translesion synthesis / Activation of ATR in response to replication stress / response to cadmium ion / DNA polymerase binding / positive regulation of B cell proliferation / epithelial cell differentiation / positive regulation of DNA repair / Translesion synthesis by REV1 / Translesion synthesis by POLK / Translesion synthesis by POLI / Gap-filling DNA repair synthesis and ligation in GG-NER / base-excision repair, gap-filling / TP53 Regulates Transcription of Genes Involved in G2 Cell Cycle Arrest / replication fork / positive regulation of DNA replication / male germ cell nucleus / liver regeneration / nuclear estrogen receptor binding / Recognition of DNA damage by PCNA-containing replication complex / Termination of translesion DNA synthesis / Translesion Synthesis by POLH / HDR through Homologous Recombination (HRR) / G2/M DNA damage checkpoint / Dual Incision in GG-NER / receptor tyrosine kinase binding / DNA-templated DNA replication / cellular response to hydrogen peroxide / Dual incision in TC-NER / Gap-filling DNA repair synthesis and ligation in TC-NER / cellular response to UV / cellular response to xenobiotic stimulus / E3 ubiquitin ligases ubiquitinate target proteins / response to estradiol / heart development / Processing of DNA double-strand break ends / DNA replication / Regulation of TP53 Activity through Phosphorylation / cell population proliferation / chromosome, telomeric region / damaged DNA binding / nuclear body / DNA repair / centrosome / chromatin binding / protein-containing complex binding Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
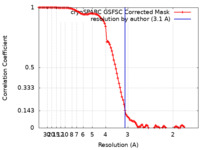
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | ||||||||||||
 Authors Authors | Wang F / He Q / Li H | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2024 Journal: Nat Struct Mol Biol / Year: 2024Title: The human ATAD5 has evolved unique structural elements to function exclusively as a PCNA unloader. Authors: Feng Wang / Qing He / Nina Y Yao / Michael E O'Donnell / Huilin Li /  Abstract: Humans have three different proliferating cell nuclear antigen (PCNA) clamp-loading complexes: RFC and CTF18-RFC load PCNA onto DNA, but ATAD5-RFC can only unload PCNA from DNA. The underlying ...Humans have three different proliferating cell nuclear antigen (PCNA) clamp-loading complexes: RFC and CTF18-RFC load PCNA onto DNA, but ATAD5-RFC can only unload PCNA from DNA. The underlying structural basis of ATAD5-RFC unloading is unknown. We show here that ATAD5 has two unique locking loops that appear to tie the complex into a rigid structure, and together with a domain that plugs the DNA-binding chamber, prevent conformation changes required for DNA binding, likely explaining why ATAD5-RFC is exclusively a PCNA unloader. These features are conserved in the yeast PCNA unloader Elg1-RFC. We observe intermediates in which PCNA bound to ATAD5-RFC exists as a closed planar ring, a cracked spiral or a gapped spiral. Surprisingly, ATAD5-RFC can open a PCNA gap between PCNA protomers 2 and 3, different from the PCNA protomers 1 and 3 gap observed in all previously characterized clamp loaders. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_42288.map.gz emd_42288.map.gz | 116.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-42288-v30.xml emd-42288-v30.xml emd-42288.xml emd-42288.xml | 26.6 KB 26.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_42288_fsc.xml emd_42288_fsc.xml | 10.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_42288.png emd_42288.png | 65.6 KB | ||
| Filedesc metadata |  emd-42288.cif.gz emd-42288.cif.gz | 7.7 KB | ||
| Others |  emd_42288_additional_1.map.gz emd_42288_additional_1.map.gz emd_42288_additional_2.map.gz emd_42288_additional_2.map.gz emd_42288_half_map_1.map.gz emd_42288_half_map_1.map.gz emd_42288_half_map_2.map.gz emd_42288_half_map_2.map.gz | 61.9 MB 111.1 MB 116 MB 116 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-42288 http://ftp.pdbj.org/pub/emdb/structures/EMD-42288 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42288 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42288 | HTTPS FTP |
-Validation report
| Summary document |  emd_42288_validation.pdf.gz emd_42288_validation.pdf.gz | 886.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_42288_full_validation.pdf.gz emd_42288_full_validation.pdf.gz | 886 KB | Display | |
| Data in XML |  emd_42288_validation.xml.gz emd_42288_validation.xml.gz | 19.2 KB | Display | |
| Data in CIF |  emd_42288_validation.cif.gz emd_42288_validation.cif.gz | 24.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42288 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42288 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42288 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42288 | HTTPS FTP |
-Related structure data
| Related structure data |  8ui8MC  8ui7C  8ui9C  8uiiC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_42288.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_42288.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Final sharpened Cryo-EM map | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.828 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Final Unsharpened Cryo-EM map
| File | emd_42288_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Final Unsharpened Cryo-EM map | ||||||||||||
| Projections & Slices |
| ||||||||||||
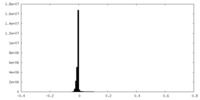
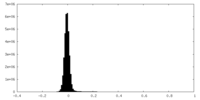
| Density Histograms |
-Additional map: Sharpened Cryo-EM map by DeepEMhancer
| File | emd_42288_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened Cryo-EM map by DeepEMhancer | ||||||||||||
| Projections & Slices |
| ||||||||||||
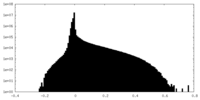
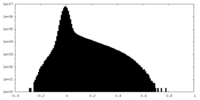
| Density Histograms |
-Half map: Half Map A
| File | emd_42288_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
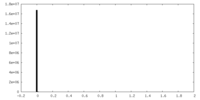
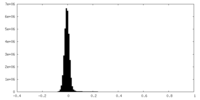
| Density Histograms |
-Half map: Half Map B
| File | emd_42288_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
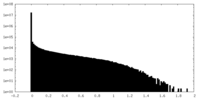
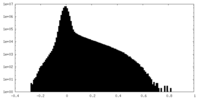
| Density Histograms |
- Sample components
Sample components
+Entire : ATAD5-RFC-closed PCNA
+Supramolecule #1: ATAD5-RFC-closed PCNA
+Macromolecule #1: ATPase family AAA domain-containing protein 5
+Macromolecule #2: Replication factor C subunit 2
+Macromolecule #3: Replication factor C subunit 5
+Macromolecule #4: Replication factor C subunit 4
+Macromolecule #5: Replication factor C subunit 3
+Macromolecule #6: Proliferating cell nuclear antigen
+Macromolecule #7: MAGNESIUM ION
+Macromolecule #8: PHOSPHOTHIOPHOSPHORIC ACID-ADENYLATE ESTER
+Macromolecule #9: ADENOSINE-5'-DIPHOSPHATE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.0 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: DIFFRACTION / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
 Movie
Movie Controller
Controller
























 Z
Z Y
Y X
X