+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of REGN7663-Fab bound CXCR4 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GPCR / chemokine receptor / antibody / fab / SIGNALING PROTEIN-IMMUNE SYSTEM complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationC-X-C motif chemokine 12 receptor activity / regulation of viral process / positive regulation of vascular wound healing / positive regulation of macrophage migration inhibitory factor signaling pathway / neuron recognition / telencephalon cell migration / positive regulation of mesenchymal stem cell migration / response to tacrolimus / response to ultrasound / Specification of primordial germ cells ...C-X-C motif chemokine 12 receptor activity / regulation of viral process / positive regulation of vascular wound healing / positive regulation of macrophage migration inhibitory factor signaling pathway / neuron recognition / telencephalon cell migration / positive regulation of mesenchymal stem cell migration / response to tacrolimus / response to ultrasound / Specification of primordial germ cells / CXCL12-activated CXCR4 signaling pathway / myosin light chain binding / myelin maintenance / C-X-C chemokine receptor activity / regulation of programmed cell death / positive regulation of vasculature development / endothelial tube morphogenesis / endothelial cell differentiation / Signaling by ROBO receptors / regulation of chemotaxis / positive regulation of dendrite extension / Formation of definitive endoderm / C-C chemokine receptor activity / positive regulation of chemotaxis / Developmental Lineage of Pancreatic Acinar Cells / C-C chemokine binding / Chemokine receptors bind chemokines / anchoring junction / dendritic cell chemotaxis / epithelial cell development / cellular response to cytokine stimulus / small molecule binding / cell leading edge / detection of temperature stimulus involved in sensory perception of pain / positive regulation of oligodendrocyte differentiation / regulation of calcium ion transport / Binding and entry of HIV virion / detection of mechanical stimulus involved in sensory perception of pain / regulation of cell adhesion / coreceptor activity / cardiac muscle contraction / neurogenesis / ubiquitin binding / response to activity / cell chemotaxis / calcium-mediated signaling / G protein-coupled receptor activity / brain development / response to virus / neuron migration / late endosome / cellular response to xenobiotic stimulus / actin binding / positive regulation of cold-induced thermogenesis / virus receptor activity / positive regulation of cytosolic calcium ion concentration / cytoplasmic vesicle / G alpha (i) signalling events / early endosome / lysosome / response to hypoxia / immune response / positive regulation of cell migration / G protein-coupled receptor signaling pathway / inflammatory response / external side of plasma membrane / apoptotic process / ubiquitin protein ligase binding / cell surface / protein-containing complex / extracellular exosome / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Saotome K / McGoldrick LL / Franklin MC | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2025 Journal: Nat Struct Mol Biol / Year: 2025Title: Structural insights into CXCR4 modulation and oligomerization. Authors: Kei Saotome / Luke L McGoldrick / Jo-Hao Ho / Trudy F Ramlall / Sweta Shah / Michael J Moore / Jee Hae Kim / Raymond Leidich / William C Olson / Matthew C Franklin /  Abstract: Activation of the chemokine receptor CXCR4 by its chemokine ligand CXCL12 regulates diverse cellular processes. Previously reported crystal structures of CXCR4 revealed the architecture of an ...Activation of the chemokine receptor CXCR4 by its chemokine ligand CXCL12 regulates diverse cellular processes. Previously reported crystal structures of CXCR4 revealed the architecture of an inactive, homodimeric receptor. However, many structural aspects of CXCR4 remain poorly understood. Here, we use cryo-electron microscopy to investigate various modes of human CXCR4 regulation. CXCL12 activates CXCR4 by inserting its N terminus deep into the CXCR4 orthosteric pocket. The binding of US Food and Drug Administration-approved antagonist AMD3100 is stabilized by electrostatic interactions with acidic residues in the seven-transmembrane-helix bundle. A potent antibody blocker, REGN7663, binds across the extracellular face of CXCR4 and inserts its complementarity-determining region H3 loop into the orthosteric pocket. Trimeric and tetrameric structures of CXCR4 reveal modes of G-protein-coupled receptor oligomerization. We show that CXCR4 adopts distinct subunit conformations in trimeric and tetrameric assemblies, highlighting how oligomerization could allosterically regulate chemokine receptor function. #1:  Journal: Biorxiv / Year: 2024 Journal: Biorxiv / Year: 2024Title: Structural insights into CXCR4 modulation and oligomerization Authors: Saotome K / McGoldrick LL / Ho J / Ramlall T / Shah S / Moore MJ / Kim JH / Leidich R / Olson WC / Franklin MC | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41892.map.gz emd_41892.map.gz | 97.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41892-v30.xml emd-41892-v30.xml emd-41892.xml emd-41892.xml | 21.9 KB 21.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_41892.png emd_41892.png | 114.4 KB | ||
| Filedesc metadata |  emd-41892.cif.gz emd-41892.cif.gz | 6.8 KB | ||
| Others |  emd_41892_half_map_1.map.gz emd_41892_half_map_1.map.gz emd_41892_half_map_2.map.gz emd_41892_half_map_2.map.gz | 95.6 MB 95.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41892 http://ftp.pdbj.org/pub/emdb/structures/EMD-41892 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41892 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41892 | HTTPS FTP |
-Validation report
| Summary document |  emd_41892_validation.pdf.gz emd_41892_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_41892_full_validation.pdf.gz emd_41892_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_41892_validation.xml.gz emd_41892_validation.xml.gz | 13.2 KB | Display | |
| Data in CIF |  emd_41892_validation.cif.gz emd_41892_validation.cif.gz | 15.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41892 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41892 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41892 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41892 | HTTPS FTP |
-Related structure data
| Related structure data |  8u4rMC  8u4nC  8u4oC  8u4pC  8u4qC  8u4sC  8u4tC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_41892.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41892.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.85 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_41892_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
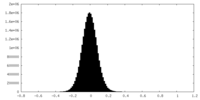
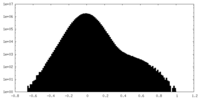
| Density Histograms |
-Half map: #2
| File | emd_41892_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
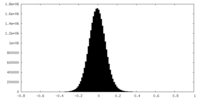
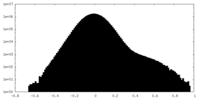
| Density Histograms |
- Sample components
Sample components
-Entire : REGN7663 Fab-bound CXCR4
| Entire | Name: REGN7663 Fab-bound CXCR4 |
|---|---|
| Components |
|
-Supramolecule #1: REGN7663 Fab-bound CXCR4
| Supramolecule | Name: REGN7663 Fab-bound CXCR4 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: REGN7663 Fab light chain
| Macromolecule | Name: REGN7663 Fab light chain / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 24.185904 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DVVMTQSPLS LPVTLGQPAS ISCRSSQSLV YTDGNTYLNW FQQRPGQSPR RLIYKVSNRD SGVPDRFSGS GSGTDFTLKI SRVEAEDVG FYYCMQNTHW PLTFGGGTKV EIKRTVAAPS VFIFPPSDEQ LKSGTASVVC LLNNFYPREA KVQWKVDNAL Q SGNSQESV ...String: DVVMTQSPLS LPVTLGQPAS ISCRSSQSLV YTDGNTYLNW FQQRPGQSPR RLIYKVSNRD SGVPDRFSGS GSGTDFTLKI SRVEAEDVG FYYCMQNTHW PLTFGGGTKV EIKRTVAAPS VFIFPPSDEQ LKSGTASVVC LLNNFYPREA KVQWKVDNAL Q SGNSQESV TEQDSKDSTY SLSSTLTLSK ADYEKHKVYA CEVTHQGLSS PVTKSFNRGE C |
-Macromolecule #2: REGN7663 Fab heavy chain
| Macromolecule | Name: REGN7663 Fab heavy chain / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 25.799934 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QVQLVQSGAE VKKPGASVKV SCKASGYTFT SYGISWVRQA PGQGIEWMGW ISTYNGNRNY AQKVQGRVTM TTDRSTSTAY MDLRSLRSD DTAVYYCARH GITGARNYYY HYGMDVWGQG TTVTVSSAST KGPSVFPLAP CSRSTSESTA ALGCLVKDYF P EPVTVSWN ...String: QVQLVQSGAE VKKPGASVKV SCKASGYTFT SYGISWVRQA PGQGIEWMGW ISTYNGNRNY AQKVQGRVTM TTDRSTSTAY MDLRSLRSD DTAVYYCARH GITGARNYYY HYGMDVWGQG TTVTVSSAST KGPSVFPLAP CSRSTSESTA ALGCLVKDYF P EPVTVSWN SGALTSGVHT FPAVLQSSGL YSLSSVVTVP SSSLGTKTYT CNVDHKPSNT KVDKRVESKY GPPCPPCPAP PV |
-Macromolecule #3: C-X-C chemokine receptor type 4
| Macromolecule | Name: C-X-C chemokine receptor type 4 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 71.063609 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKTIIALSYI FCLVFAGAPE GISIYTSDNY TEEMGSGDYD SMKEPCFREE NANFNKIFLP TIYSIIFLTG IVGNGLVILV MGYQKKLRS MTDKYRLHLS VADLLFVITL PFWAVDAVAN WYFGNFLCKA VHVIYTVSLY SSVLILAFIS LDRYLAIVHA T NSQRPRKL ...String: MKTIIALSYI FCLVFAGAPE GISIYTSDNY TEEMGSGDYD SMKEPCFREE NANFNKIFLP TIYSIIFLTG IVGNGLVILV MGYQKKLRS MTDKYRLHLS VADLLFVITL PFWAVDAVAN WYFGNFLCKA VHVIYTVSLY SSVLILAFIS LDRYLAIVHA T NSQRPRKL LAEKVVYVGV WIPALLLTIP DFIFANVSEA DDRYICDRFY PNDLWVVVFQ FQHIMVGLIL PGIVILSCYC II ISKLSHS KGHQKRKALK TTVILILAFF ACWLPYYIGI SIDSFILLEI IKQGCEFENT VHKWISITEA LAFFHCCLNP ILY AFLGAK FKTSAQHALT SVSRGSSLKI LSKGKRGGHS SVSTESESSS FHSSGRPLEV LFQGPGGGGS VSKGEELFTG VVPI LVELD GDVNGHKFSV SGEGEGDATY GKLTLKFICT TGKLPVPWPT LVTTLTYGVQ CFSRYPDHMK QHDFFKSAMP EGYVQ ERTI FFKDDGNYKT RAEVKFEGDT LVNRIELKGI DFKEDGNILG HKLEYNYNSH NVYIMADKQK NGIKVNFKIR HNIEDG SVQ LADHYQQNTP IGDGPVLLPD NHYLSTQSKL SKDPNEKRDH MVLLEFVTAA GITLGMDELY KDYKDDDDK UniProtKB: C-X-C chemokine receptor type 4 |
-Macromolecule #4: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 4 / Number of copies: 1 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.1 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 102810 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller
























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)