[English] 日本語
 Yorodumi
Yorodumi- EMDB-39396: Localized reconstruction of Hepatitis B virus surface antigen dim... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Localized reconstruction of Hepatitis B virus surface antigen dimer in the subviral particle with D2 symmetry from dataset A0 | ||||||||||||||||||||||||
 Map data Map data | |||||||||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||||||||
 Keywords Keywords | Surface antigen / Localized reconstruction / VIRAL PROTEIN | ||||||||||||||||||||||||
| Function / homology | Large envelope protein S / Major surface antigen from hepadnavirus / caveolin-mediated endocytosis of virus by host cell / fusion of virus membrane with host endosome membrane / viral envelope / virion attachment to host cell / virion membrane / membrane / Large envelope protein Function and homology information Function and homology information | ||||||||||||||||||||||||
| Biological species |  Hepatitis B virus ayw/China/Tibet127/2002 Hepatitis B virus ayw/China/Tibet127/2002 | ||||||||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.7 Å | ||||||||||||||||||||||||
 Authors Authors | Wang T / Cao L / Mu A / Wang Q / Rao ZH | ||||||||||||||||||||||||
| Funding support |  China, 7 items China, 7 items
| ||||||||||||||||||||||||
 Citation Citation |  Journal: Science / Year: 2024 Journal: Science / Year: 2024Title: Inherent symmetry and flexibility in hepatitis B virus subviral particles. Authors: Quan Wang / Tao Wang / Lin Cao / An Mu / Sheng Fu / Peipei Wang / Yan Gao / Wenxin Ji / Zhenyu Liu / Zhanqiang Du / Luke W Guddat / Wenchi Zhang / Shuang Li / Xuemei Li / Zhiyong Lou / ...Authors: Quan Wang / Tao Wang / Lin Cao / An Mu / Sheng Fu / Peipei Wang / Yan Gao / Wenxin Ji / Zhenyu Liu / Zhanqiang Du / Luke W Guddat / Wenchi Zhang / Shuang Li / Xuemei Li / Zhiyong Lou / Xiangxi Wang / Zhongyu Hu / Zihe Rao /   Abstract: Chronic hepatitis B virus (HBV) infection poses a major global health challenge with massive morbidity and mortality. Despite a preventive vaccine, current treatments provide limited virus clearance, ...Chronic hepatitis B virus (HBV) infection poses a major global health challenge with massive morbidity and mortality. Despite a preventive vaccine, current treatments provide limited virus clearance, necessitating lifelong commitment. The HBV surface antigen (HBsAg) is crucial for diagnosis and prognosis, yet its high-resolution structure and assembly on the virus envelope remain elusive. Utilizing extensive datasets and advanced cryo-electron microscopy analysis, we present structural insights into HBsAg at a near-atomic resolution of 3.7 angstroms. HBsAg homodimers assemble into subviral particles with - and -like quasisymmetry, elucidating the dense-packing rules and structural adaptability of HBsAg. These findings provide insights into how HBsAg assembles into higher-order filaments and interacts with the capsid to form virions. | ||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_39396.map.gz emd_39396.map.gz | 84.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-39396-v30.xml emd-39396-v30.xml emd-39396.xml emd-39396.xml | 21.8 KB 21.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_39396_fsc.xml emd_39396_fsc.xml | 10.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_39396.png emd_39396.png | 50.5 KB | ||
| Masks |  emd_39396_msk_1.map emd_39396_msk_1.map | 91.1 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-39396.cif.gz emd-39396.cif.gz | 6.4 KB | ||
| Others |  emd_39396_additional_1.map.gz emd_39396_additional_1.map.gz emd_39396_half_map_1.map.gz emd_39396_half_map_1.map.gz emd_39396_half_map_2.map.gz emd_39396_half_map_2.map.gz | 20.8 MB 82.2 MB 82.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-39396 http://ftp.pdbj.org/pub/emdb/structures/EMD-39396 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39396 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39396 | HTTPS FTP |
-Validation report
| Summary document |  emd_39396_validation.pdf.gz emd_39396_validation.pdf.gz | 658.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_39396_full_validation.pdf.gz emd_39396_full_validation.pdf.gz | 658 KB | Display | |
| Data in XML |  emd_39396_validation.xml.gz emd_39396_validation.xml.gz | 17.9 KB | Display | |
| Data in CIF |  emd_39396_validation.cif.gz emd_39396_validation.cif.gz | 23.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39396 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39396 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39396 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39396 | HTTPS FTP |
-Related structure data
| Related structure data |  8ymkMC  8ymjC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_39396.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_39396.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||
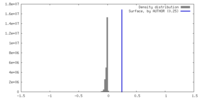
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_39396_msk_1.map emd_39396_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
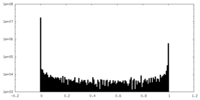
| Density Histograms |
-Additional map: Localized reconstruction from dataset A, generated by CryoSPARC...
| File | emd_39396_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Localized reconstruction from dataset A, generated by CryoSPARC local refinement job, and then resampled on the dataset A0 map (main map of this entry) in UCSF Chimera. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_39396_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_39396_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Hepatitis B virus ayw/China/Tibet127/2002
| Entire | Name:  Hepatitis B virus ayw/China/Tibet127/2002 Hepatitis B virus ayw/China/Tibet127/2002 |
|---|---|
| Components |
|
-Supramolecule #1: Hepatitis B virus ayw/China/Tibet127/2002
| Supramolecule | Name: Hepatitis B virus ayw/China/Tibet127/2002 / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 489469 / Sci species name: Hepatitis B virus ayw/China/Tibet127/2002 / Virus type: VIRUS-LIKE PARTICLE / Virus isolate: SEROTYPE / Virus enveloped: Yes / Virus empty: Yes |
|---|
-Macromolecule #1: Isoform S of Large envelope protein
| Macromolecule | Name: Isoform S of Large envelope protein / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Hepatitis B virus ayw/China/Tibet127/2002 / Strain: isolate China/Tibet127/2002 Hepatitis B virus ayw/China/Tibet127/2002 / Strain: isolate China/Tibet127/2002 |
| Molecular weight | Theoretical: 25.408059 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MENITSGFLG PLLVLQAGFF LLTRILTIPQ SLDSWWTSLN FLGGTTVCLG QNSQSPISNH SPTSCPPTCP GYRWMCLRRF IIFLFILLL CLIFLLVLLD YQGMLPVCPL IPGSSTTSTG PCRTCTTPAQ GTSMYPSCCC TKPSDGNCTC IPIPSSWAFG K FLWEWASA ...String: MENITSGFLG PLLVLQAGFF LLTRILTIPQ SLDSWWTSLN FLGGTTVCLG QNSQSPISNH SPTSCPPTCP GYRWMCLRRF IIFLFILLL CLIFLLVLLD YQGMLPVCPL IPGSSTTSTG PCRTCTTPAQ GTSMYPSCCC TKPSDGNCTC IPIPSSWAFG K FLWEWASA RFSWLSLLVP FVQWFVGLSP TVWLSVIWMM WYWGPSLYSI LSPFLPLLPI FFCLWVYI UniProtKB: Large envelope protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: Quantifoil R0.6/1 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: OTHER |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Digitization - Dimensions - Width: 5760 pixel / Digitization - Dimensions - Height: 4092 pixel / Number grids imaged: 50 / Number real images: 157206 / Average exposure time: 3.0 sec. / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.8 µm / Nominal defocus min: 1.2 µm / Nominal magnification: 22500 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: A / Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
| Output model |  PDB-8ymk: |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)