[English] 日本語
 Yorodumi
Yorodumi- EMDB-32820: Cryo-EM structure of the adhesion GPCR ADGRF1(H565A/T567A) in com... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the adhesion GPCR ADGRF1(H565A/T567A) in complex with miniGi | ||||||||||||||||||
 Map data Map data | |||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | Adhesion GPCR / ADGRF1 / G protein / Complex / Signal transduction / MEMBRANE PROTEIN | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationenergy reserve metabolic process / fat cell differentiation / regulation of lipid metabolic process / T cell migration / Adenylate cyclase inhibitory pathway / positive regulation of protein localization to cell cortex / regulation of cAMP-mediated signaling / D2 dopamine receptor binding / G protein-coupled serotonin receptor binding / regulation of mitotic spindle organization ...energy reserve metabolic process / fat cell differentiation / regulation of lipid metabolic process / T cell migration / Adenylate cyclase inhibitory pathway / positive regulation of protein localization to cell cortex / regulation of cAMP-mediated signaling / D2 dopamine receptor binding / G protein-coupled serotonin receptor binding / regulation of mitotic spindle organization / synapse assembly / cellular response to forskolin / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / Regulation of insulin secretion / G protein-coupled receptor binding / G protein-coupled receptor activity / G-protein beta/gamma-subunit complex binding / adenylate cyclase-activating G protein-coupled receptor signaling pathway / Olfactory Signaling Pathway / Activation of the phototransduction cascade / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / G-protein activation / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / memory / Prostacyclin signalling through prostacyclin receptor / response to peptide hormone / Glucagon signaling in metabolic regulation / G beta:gamma signalling through CDC42 / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / photoreceptor disc membrane / Glucagon-type ligand receptors / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / G alpha (z) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / cellular response to catecholamine stimulus / ADORA2B mediated anti-inflammatory cytokines production / sensory perception of taste / ADP signalling through P2Y purinoceptor 1 / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / GDP binding / cellular response to prostaglandin E stimulus / neuron projection development / Inactivation, recovery and regulation of the phototransduction cascade / G-protein beta-subunit binding / heterotrimeric G-protein complex / G alpha (12/13) signalling events / extracellular vesicle / signaling receptor complex adaptor activity / Thrombin signalling through proteinase activated receptors (PARs) / GTPase binding / retina development in camera-type eye / Ca2+ pathway / phospholipase C-activating G protein-coupled receptor signaling pathway / cell cortex / midbody / G alpha (i) signalling events / fibroblast proliferation / cytoplasmic vesicle / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / G alpha (s) signalling events / G alpha (q) signalling events / Ras protein signal transduction / cell population proliferation / Extra-nuclear estrogen signaling / cell surface receptor signaling pathway / G protein-coupled receptor signaling pathway / cell division / lysosomal membrane / GTPase activity / centrosome / synapse / protein-containing complex binding / nucleolus / GTP binding / magnesium ion binding / signal transduction / extracellular exosome / extracellular region / nucleoplasm / membrane / plasma membrane / cytosol / cytoplasm Similarity search - Function | ||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | ||||||||||||||||||
 Authors Authors | Qu X / Qiu N | ||||||||||||||||||
| Funding support |  China, 5 items China, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Structural basis of tethered agonism of the adhesion GPCRs ADGRD1 and ADGRF1. Authors: Xiangli Qu / Na Qiu / Mu Wang / Bingjie Zhang / Juan Du / Zhiwei Zhong / Wei Xu / Xiaojing Chu / Limin Ma / Cuiying Yi / Shuo Han / Wenqing Shui / Qiang Zhao / Beili Wu /  Abstract: Adhesion G protein-coupled receptors (aGPCRs) are essential for a variety of physiological processes such as immune responses, organ development, cellular communication, proliferation and homeostasis. ...Adhesion G protein-coupled receptors (aGPCRs) are essential for a variety of physiological processes such as immune responses, organ development, cellular communication, proliferation and homeostasis. An intrinsic manner of activation that involves a tethered agonist in the N-terminal region of the receptor has been proposed for the aGPCRs, but its molecular mechanism remains elusive. Here we report the G protein-bound structures of ADGRD1 and ADGRF1, which exhibit many unique features with regard to the tethered agonism. The stalk region that proceeds the first transmembrane helix acts as the tethered agonist by forming extensive interactions with the transmembrane domain; these interactions are mostly conserved in ADGRD1 and ADGRF1, suggesting that a common stalk-transmembrane domain interaction pattern is shared by members of the aGPCR family. A similar stalk binding mode is observed in the structure of autoproteolysis-deficient ADGRF1, supporting a cleavage-independent manner of receptor activation. The stalk-induced activation is facilitated by a cascade of inter-helix interaction cores that are conserved in positions but show sequence variability in these two aGPCRs. Furthermore, the intracellular region of ADGRF1 contains a specific lipid-binding site, which proves to be functionally important and may serve as the recognition site for the previously discovered endogenous ADGRF1 ligand synaptamide. These findings highlight the diversity and complexity of the signal transduction mechanisms of the aGPCRs. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_32820.map.gz emd_32820.map.gz | 5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-32820-v30.xml emd-32820-v30.xml emd-32820.xml emd-32820.xml | 15.5 KB 15.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_32820.png emd_32820.png | 63.6 KB | ||
| Filedesc metadata |  emd-32820.cif.gz emd-32820.cif.gz | 6.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-32820 http://ftp.pdbj.org/pub/emdb/structures/EMD-32820 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32820 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32820 | HTTPS FTP |
-Validation report
| Summary document |  emd_32820_validation.pdf.gz emd_32820_validation.pdf.gz | 360.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_32820_full_validation.pdf.gz emd_32820_full_validation.pdf.gz | 359.7 KB | Display | |
| Data in XML |  emd_32820_validation.xml.gz emd_32820_validation.xml.gz | 6 KB | Display | |
| Data in CIF |  emd_32820_validation.cif.gz emd_32820_validation.cif.gz | 7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32820 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32820 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32820 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32820 | HTTPS FTP |
-Related structure data
| Related structure data |  7wu5MC  7wu2C  7wu3C  7wu4C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_32820.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_32820.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.071 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : The adhesion GPCR ADGRF1(H565A/T567A) in complex with miniGi
| Entire | Name: The adhesion GPCR ADGRF1(H565A/T567A) in complex with miniGi |
|---|---|
| Components |
|
-Supramolecule #1: The adhesion GPCR ADGRF1(H565A/T567A) in complex with miniGi
| Supramolecule | Name: The adhesion GPCR ADGRF1(H565A/T567A) in complex with miniGi type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Guanine nucleotide-binding protein G(i) subunit alpha-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(i) subunit alpha-1 type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 27.563354 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGHHHHHHEN LYFQGTLSAE DKAAVERSKM IDRNLREDGE KAAREVKLLL LGADNSGKST IVKQMKIIHG GGGGGGGTTG IVETHFTFK DLHFKMFDVG GQRSERKKWI HCFEDVAAII FCVDLSDYNR MHESMKLFDS ICNNKWFTDT SIILFLNKKD L FEEKIKKS ...String: MGHHHHHHEN LYFQGTLSAE DKAAVERSKM IDRNLREDGE KAAREVKLLL LGADNSGKST IVKQMKIIHG GGGGGGGTTG IVETHFTFK DLHFKMFDVG GQRSERKKWI HCFEDVAAII FCVDLSDYNR MHESMKLFDS ICNNKWFTDT SIILFLNKKD L FEEKIKKS PLTICYQEYA GSNTYEEAAA YIQCQFEDLN KRKDTKEIYT HFTCATDTKN AQFIFDAVTD VIIKNNLKDC GL F UniProtKB: G protein subunit alpha i1, Guanine nucleotide-binding protein G(i) subunit alpha-1 |
-Macromolecule #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 38.744371 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MHHHHHHGSL LQSELDQLRQ EAEQLKNQIR DARKACADAT LSQITNNIDP VGRIQMRTRR TLRGHLAKIY AMHWGTDSRL LVSASQDGK LIIWDSYTTN KVHAIPLRSS WVMTCAYAPS GNYVACGGLD NICSIYNLKT REGNVRVSRE LAGHTGYLSC C RFLDDNQI ...String: MHHHHHHGSL LQSELDQLRQ EAEQLKNQIR DARKACADAT LSQITNNIDP VGRIQMRTRR TLRGHLAKIY AMHWGTDSRL LVSASQDGK LIIWDSYTTN KVHAIPLRSS WVMTCAYAPS GNYVACGGLD NICSIYNLKT REGNVRVSRE LAGHTGYLSC C RFLDDNQI VTSSGDTTCA LWDIETGQQT TTFTGHTGDV MSLSLAPDTR LFVSGACDAS AKLWDVREGM CRQTFTGHES DI NAICFFP NGNAFATGSD DATCRLFDLR ADQELMTYSH DNIICGITSV SFSKSGRLLL AGYDDFNCNV WDALKADRAG VLA GHDNRV SCLGVTDDGM AVATGSWDSF LKIWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #4: Adhesion G-protein coupled receptor F1
| Macromolecule | Name: Adhesion G-protein coupled receptor F1 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 74.513406 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKTIIALSYI FCLVFADYKD DDDGAPVFGK AQCNDIVFGF GSKDDEYTLP CSSGYRGNIT AKCESSGWQV IRETCVLSLL EELNKNFSM IVGNATEAAV SSFVQNLSVI IRQNPSTTVG NLASVVSILS NISSLSLASH FRVSNSTMED VISIADNILN S ASVTNWTV ...String: MKTIIALSYI FCLVFADYKD DDDGAPVFGK AQCNDIVFGF GSKDDEYTLP CSSGYRGNIT AKCESSGWQV IRETCVLSLL EELNKNFSM IVGNATEAAV SSFVQNLSVI IRQNPSTTVG NLASVVSILS NISSLSLASH FRVSNSTMED VISIADNILN S ASVTNWTV LLREEKYASS RLLETLENIS TLVPPTALPL NFSRKFIDWK GIPVNKSQLK RGYSYQIKMC PQNTSIPIRG RV LIGSDQF QRSLPETIIS MASLTLGNIL PVSKNGNAQV NGPVISTVIQ NYSINEVFLF FSKIESNLSQ PHCVFWDFSH LQW NDAGCH LVNETQDIVT CQCTALASFS ILMSPFVPST IFPVVKWITY VGLGISIGSL ILCLIIEALF WKQIKKSQTS HTRR ICMVN IALSLLIADV WFIVGATVDT TVNPSGVCTA AVFFTHFFYL SLFFWMLMLG ILLAYRIILV FHHMAQHLMM AVGFC LGYG CPLIISVITI AVTQPSNTYK RKDVCWLNWS NGSKPLLAFV VPALAIVAVN FVVVLLVLTK LWRPTVGERL SRDDKA TII RVGKSLLILT PLLGLTWGFG IGTIVDSQNL AWHVIFALLN AFQGFFILCF GILLDSKLRQ LLFNKLSALS SWKEFLE VL FQGPWSHPQF EKGGGSGGGS GGSAWSHPQF EK UniProtKB: Adhesion G-protein coupled receptor F1 |
-Macromolecule #5: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 5 / Number of copies: 1 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Macromolecule #6: [(2~{R})-2-oxidanyl-3-[oxidanyl-[2-(trimethyl-$l^{4}-azanyl)ethox...
| Macromolecule | Name: [(2~{R})-2-oxidanyl-3-[oxidanyl-[2-(trimethyl-$l^{4}-azanyl)ethoxy]phosphoryl]oxy-propyl] hexadecanoate type: ligand / ID: 6 / Number of copies: 1 / Formula: K6G |
|---|---|
| Molecular weight | Theoretical: 496.638 Da |
| Chemical component information | 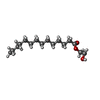 ChemComp-K6G: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 70.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: INSILICO MODEL |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.0 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 799431 |
| Initial angle assignment | Type: OTHER |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




















