+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-30533 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
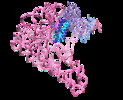
| Title | Cryo-EM structure of a pre-catalytic group II intron | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | group II intron / RNA-protein complex / pre-catalytic / TRANSFERASE-RNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationintron homing / RNA-directed DNA polymerase / mRNA processing / RNA-directed DNA polymerase activity / endonuclease activity / Hydrolases; Acting on ester bonds Similarity search - Function | |||||||||
| Biological species |  Lactococcus lactis subsp. cremoris (lactic acid bacteria) Lactococcus lactis subsp. cremoris (lactic acid bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 5.0 Å | |||||||||
 Authors Authors | Liu N / Dong XL | |||||||||
| Funding support |  China, China,  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2020 Journal: Nucleic Acids Res / Year: 2020Title: Exon and protein positioning in a pre-catalytic group II intron RNP primed for splicing. Authors: Nan Liu / Xiaolong Dong / Cuixia Hu / Jianwei Zeng / Jiawei Wang / Jia Wang / Hong-Wei Wang / Marlene Belfort /   Abstract: Group II introns are the putative progenitors of nuclear spliceosomal introns and use the same two-step splicing pathway. In the cell, the intron RNA forms a ribonucleoprotein (RNP) complex with the ...Group II introns are the putative progenitors of nuclear spliceosomal introns and use the same two-step splicing pathway. In the cell, the intron RNA forms a ribonucleoprotein (RNP) complex with the intron-encoded protein (IEP), which is essential for splicing. Although structures of spliced group II intron RNAs and RNP complexes have been characterized, structural insights into the splicing process remain enigmatic due to lack of pre-catalytic structural models. Here, we report two cryo-EM structures of endogenously produced group II intron RNPs trapped in their pre-catalytic state. Comparison of the catalytically activated precursor RNP to its previously reported spliced counterpart allowed identification of key structural rearrangements accompanying splicing, including a remodeled active site and engagement of the exons. Importantly, altered RNA-protein interactions were observed upon splicing among the RNP complexes. Furthermore, analysis of the catalytically inert precursor RNP demonstrated the structural impact of the formation of the active site on RNP architecture. Taken together, our results not only fill a gap in understanding the structural basis of IEP-assisted group II intron splicing, but also provide parallels to evolutionarily related spliceosomal splicing. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_30533.map.gz emd_30533.map.gz | 2.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-30533-v30.xml emd-30533-v30.xml emd-30533.xml emd-30533.xml | 12.5 KB 12.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_30533.png emd_30533.png | 162.9 KB | ||
| Filedesc metadata |  emd-30533.cif.gz emd-30533.cif.gz | 5.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-30533 http://ftp.pdbj.org/pub/emdb/structures/EMD-30533 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30533 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30533 | HTTPS FTP |
-Validation report
| Summary document |  emd_30533_validation.pdf.gz emd_30533_validation.pdf.gz | 369.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_30533_full_validation.pdf.gz emd_30533_full_validation.pdf.gz | 369.2 KB | Display | |
| Data in XML |  emd_30533_validation.xml.gz emd_30533_validation.xml.gz | 5.6 KB | Display | |
| Data in CIF |  emd_30533_validation.cif.gz emd_30533_validation.cif.gz | 6.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30533 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30533 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30533 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30533 | HTTPS FTP |
-Related structure data
| Related structure data |  7d0gMC  7d0fC  7d1aC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_30533.map.gz / Format: CCP4 / Size: 23.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_30533.map.gz / Format: CCP4 / Size: 23.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.30654 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : a group II intron -- LtrB complex with its reverse transcriptase
| Entire | Name: a group II intron -- LtrB complex with its reverse transcriptase |
|---|---|
| Components |
|
-Supramolecule #1: a group II intron -- LtrB complex with its reverse transcriptase
| Supramolecule | Name: a group II intron -- LtrB complex with its reverse transcriptase type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|
-Supramolecule #2: RNA
| Supramolecule | Name: RNA / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Lactococcus lactis subsp. cremoris (lactic acid bacteria) Lactococcus lactis subsp. cremoris (lactic acid bacteria) |
-Supramolecule #3: LtrB
| Supramolecule | Name: LtrB / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  Lactococcus lactis subsp. cremoris (lactic acid bacteria) Lactococcus lactis subsp. cremoris (lactic acid bacteria) |
-Macromolecule #1: RNA (714-MER)
| Macromolecule | Name: RNA (714-MER) / type: rna / ID: 1 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:  Lactococcus lactis subsp. cremoris (lactic acid bacteria) Lactococcus lactis subsp. cremoris (lactic acid bacteria) |
| Molecular weight | Theoretical: 295.767594 KDa |
| Sequence | String: CACAUCCAUA ACGUGCGCCC AGAUAGGGUG UUAAGUCAAG UAGUUUAAGG UACUACUCUG UAAGAUAACA CAGAAAACAG CCAACCUAA CCGAAAAGCG AAAGCUGAUA CGGGAACAGA GCACGGUUGG AAAGCGAUGA GUUACCUAAA GACAAUCGGG U ACGACUGA ...String: CACAUCCAUA ACGUGCGCCC AGAUAGGGUG UUAAGUCAAG UAGUUUAAGG UACUACUCUG UAAGAUAACA CAGAAAACAG CCAACCUAA CCGAAAAGCG AAAGCUGAUA CGGGAACAGA GCACGGUUGG AAAGCGAUGA GUUACCUAAA GACAAUCGGG U ACGACUGA GUCGCAAUGU UAAUCAGAUA UAAGGUAUAA GUUGUGUUUA CUGAACGCAA GUUUCUAAUU UCGGUUAUGU GU CGAUAGA GGAAAGUGUC UGAAACCUCU AGUACAAAGA AAGGUAAGUU AUGGUUGUGG ACUUAUCUGU UAUCACCACA UUU GUACAA UCUGUAGGAG AACCUAUGGG AACGAAACGA AAGCGAUGCC GAGAAUCUGA AUUUACCAAG ACUUAACACU AACU GGGGA UACCCUAAAC AAGAAUGCCU AAUAGAAAGG AGGAAAAAGG CUAUAGCACU AGAGCUUGAA AAUCUUGCAA GGGUA CGGA GUACUCGUAG UAGUCUGAGA AGGGUAACGC CCUUUACAUG GCAAAGGGGU ACAGUUAUUG UGUACUAAAA UUAAAA AUU GAUUAGGGAG GAAAACCUCA AAAUGAAACC AACAAUGGCA AUUUUAGAAA GAAUCAGUAA AAAUUCACAA GAAAAUA UA GACGAAGUUU UUACAAGACU UUAUCGUUAU CUUUUACGUC CAGAUAUUUA UUACGUGGCG ACGCGUUGGG AAAUGGCA A UGAUAGCGAA ACAACGUAAA ACUCUUGUUG UAUGCUUUCA UUGUCAUCGU CACGUGAUUC AUAAACACAA GUGAAUUUU UACGAACGAA CAAUAACAGA AUCGUAUACU CCGAGAGGGG UACGUACGGU UCCCGAAGAG GGUGGUGCAA ACCAGUCACA GUAAUGUGA ACAAGGCGGU ACCUCCCUAC UUCACCA |
-Macromolecule #2: Group II intron-encoded protein LtrA
| Macromolecule | Name: Group II intron-encoded protein LtrA / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO / EC number: RNA-directed DNA polymerase |
|---|---|
| Source (natural) | Organism:  Lactococcus lactis subsp. cremoris (lactic acid bacteria) Lactococcus lactis subsp. cremoris (lactic acid bacteria) |
| Molecular weight | Theoretical: 70.286664 KDa |
| Recombinant expression | Organism:  Lactococcus lactis subsp. cremoris (lactic acid bacteria) Lactococcus lactis subsp. cremoris (lactic acid bacteria) |
| Sequence | String: MKPTMAILER ISKNSQENID EVFTRLYRYL LRPDIYYVAY QNLYSNKGAS TKGILDDTAD GFSEEKIKKI IQSLKDGTYY PQPVRRMYI AKKNSKKMRP LGIPTFTDKL IQEAVRIILE SIYEPVFEDV SHGFRPQRSC HTALKTIKRE FGGARWFVEG D IKGCFDNI ...String: MKPTMAILER ISKNSQENID EVFTRLYRYL LRPDIYYVAY QNLYSNKGAS TKGILDDTAD GFSEEKIKKI IQSLKDGTYY PQPVRRMYI AKKNSKKMRP LGIPTFTDKL IQEAVRIILE SIYEPVFEDV SHGFRPQRSC HTALKTIKRE FGGARWFVEG D IKGCFDNI DHVTLIGLIN LKIKDMKMSQ LIYKFLKAGY LENWQYHKTY SGTPQGGILS PLLANIYLHE LDKFVLQLKM KF DRESPER ITPEYRELHN EIKRISHRLK KLEGEEKAKV LLEYQEKRKR LPTLPCTSQT NKVLKYVRYA DDFIISVKGS KED CQWIKE QLKLFIHNKL KMELSEEKTL ITHSSQPARF LGYDIRVRRS GTIKRSGKVK KRTLNGSVEL LIPLQDKIRQ FIFD KKIAI QKKDSSWFPV HRKYLIRSTD LEIITIYNSE LRGICNYYGL ASNFNQLNYF AYLMEYSCLK TIASKHKGTL SKTIS MFKD GSGSWGIPYE IKQGKQRRYF ANFSECKSPY QFTDEISQAP VLYGYARNTL ENRLKAKCCE LCGTSDENTS YEIHHV NKV KNLKGKEKWE MAMIAKQRKT LVVCFHCHRH VIHKHK UniProtKB: Group II intron-encoded protein LtrA |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: EMDB MAP |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 5.0 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 391788 |
| Initial angle assignment | Type: PROJECTION MATCHING |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















