[English] 日本語
 Yorodumi
Yorodumi- EMDB-29873: Complete DNA elongation subcomplex of Xenopus laevis DNA polymera... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Complete DNA elongation subcomplex of Xenopus laevis DNA polymerase alpha-primase | ||||||||||||
 Map data Map data | Complete DNA elongation subcomplex of Xenopus laevis DNA polymerase alpha-primase | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Primase / DNA polymerase / chimeric RNA-DNA primer / RNA/DNA hybrid / DNA replication / DNA synthesis / REPLICATION / TRANSFERASE-DNA-RNA complex | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationalpha DNA polymerase:primase complex / primosome complex / DNA replication, synthesis of primer / lagging strand elongation / mitotic DNA replication initiation / DNA strand elongation involved in DNA replication / leading strand elongation / DNA replication initiation / double-strand break repair via nonhomologous end joining / nuclear matrix ...alpha DNA polymerase:primase complex / primosome complex / DNA replication, synthesis of primer / lagging strand elongation / mitotic DNA replication initiation / DNA strand elongation involved in DNA replication / leading strand elongation / DNA replication initiation / double-strand break repair via nonhomologous end joining / nuclear matrix / nuclear envelope / 4 iron, 4 sulfur cluster binding / DNA replication / DNA-directed DNA polymerase / DNA-directed DNA polymerase activity / nucleotide binding / chromatin binding / chromatin / nucleolus / DNA binding / nucleoplasm / nucleus / metal ion binding Similarity search - Function | ||||||||||||
| Biological species | |||||||||||||
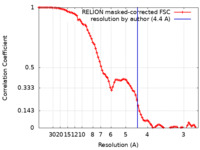
| Method | single particle reconstruction / cryo EM / Resolution: 4.4 Å | ||||||||||||
 Authors Authors | Mullins EA / Durie CL / Ohi MD / Chazin WJ / Eichman BF | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: bioRxiv / Year: 2023 Journal: bioRxiv / Year: 2023Title: A mechanistic model of primer synthesis from catalytic structures of DNA polymerase α-primase. Authors: Elwood A Mullins / Lauren E Salay / Clarissa L Durie / Noah P Bradley / Jane E Jackman / Melanie D Ohi / Walter J Chazin / Brandt F Eichman Abstract: The mechanism by which polymerase α-primase (polα-primase) synthesizes chimeric RNA-DNA primers of defined length and composition, necessary for replication fidelity and genome stability, is ...The mechanism by which polymerase α-primase (polα-primase) synthesizes chimeric RNA-DNA primers of defined length and composition, necessary for replication fidelity and genome stability, is unknown. Here, we report cryo-EM structures of polα-primase in complex with primed templates representing various stages of DNA synthesis. Our data show how interaction of the primase regulatory subunit with the primer 5'-end facilitates handoff of the primer to polα and increases polα processivity, thereby regulating both RNA and DNA composition. The structures detail how flexibility within the heterotetramer enables synthesis across two active sites and provide evidence that termination of DNA synthesis is facilitated by reduction of polα and primase affinities for the varied conformations along the chimeric primer/template duplex. Together, these findings elucidate a critical catalytic step in replication initiation and provide a comprehensive model for primer synthesis by polα-primase. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29873.map.gz emd_29873.map.gz | 40.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29873-v30.xml emd-29873-v30.xml emd-29873.xml emd-29873.xml | 29.4 KB 29.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_29873_fsc.xml emd_29873_fsc.xml | 8.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_29873.png emd_29873.png | 21 KB | ||
| Filedesc metadata |  emd-29873.cif.gz emd-29873.cif.gz | 8 KB | ||
| Others |  emd_29873_additional_1.map.gz emd_29873_additional_1.map.gz emd_29873_half_map_1.map.gz emd_29873_half_map_1.map.gz emd_29873_half_map_2.map.gz emd_29873_half_map_2.map.gz | 47 MB 40.8 MB 40.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29873 http://ftp.pdbj.org/pub/emdb/structures/EMD-29873 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29873 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29873 | HTTPS FTP |
-Validation report
| Summary document |  emd_29873_validation.pdf.gz emd_29873_validation.pdf.gz | 805.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_29873_full_validation.pdf.gz emd_29873_full_validation.pdf.gz | 805.1 KB | Display | |
| Data in XML |  emd_29873_validation.xml.gz emd_29873_validation.xml.gz | 14 KB | Display | |
| Data in CIF |  emd_29873_validation.cif.gz emd_29873_validation.cif.gz | 19.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29873 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29873 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29873 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29873 | HTTPS FTP |
-Related structure data
| Related structure data |  8g9oMC  8g99C  8g9fC  8g9lC  8g9nC  8ucuC  8ucvC  8ucwC  8v5mC  8v5nC  8v5oC  8v6gC  8v6hC  8v6iC  8v6jC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_29873.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29873.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Complete DNA elongation subcomplex of Xenopus laevis DNA polymerase alpha-primase | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.365 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Complete DNA elongation subcomplex of Xenopus laevis DNA...
| File | emd_29873_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Complete DNA elongation subcomplex of Xenopus laevis DNA polymerase alpha-primase (locally sharpened) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Complete DNA elongation subcomplex of Xenopus laevis DNA...
| File | emd_29873_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Complete DNA elongation subcomplex of Xenopus laevis DNA polymerase alpha-primase (half 1) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Complete DNA elongation subcomplex of Xenopus laevis DNA...
| File | emd_29873_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Complete DNA elongation subcomplex of Xenopus laevis DNA polymerase alpha-primase (half 2) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Polymerase alpha-primase with a DNA elongation substrate
+Supramolecule #1: Polymerase alpha-primase with a DNA elongation substrate
+Supramolecule #2: Polymerase alpha
+Supramolecule #3: Primase
+Supramolecule #4: DNA elongation substrate
+Macromolecule #1: DNA polymerase alpha catalytic subunit
+Macromolecule #2: DNA primase large subunit
+Macromolecule #3: DNA template
+Macromolecule #4: RNA-DNA primer
+Macromolecule #5: 2'-DEOXYGUANOSINE-5'-TRIPHOSPHATE
+Macromolecule #6: MAGNESIUM ION
+Macromolecule #7: IRON/SULFUR CLUSTER
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.9 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Number grids imaged: 3 / Number real images: 13641 / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 45000 |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT | ||||||||
| Output model |  PDB-8g9o: |
 Movie
Movie Controller
Controller





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)