[English] 日本語
 Yorodumi
Yorodumi- EMDB-28978: Cryo-EM structure of the human TRPV4 - RhoA in complex with 4alph... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the human TRPV4 - RhoA in complex with 4alpha-Phorbol 12,13-didecanoate | |||||||||
 Map data Map data | main | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | TRPV4 / RhoA / 4alpha-Phorbol 12 / 13-didecanoate / 4a-PDD / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationstretch-activated, monoatomic cation-selective, calcium channel activity / blood vessel endothelial cell delamination / osmosensor activity / vasopressin secretion / positive regulation of striated muscle contraction / positive regulation of macrophage inflammatory protein 1 alpha production / calcium ion import into cytosol / negative regulation of brown fat cell differentiation / positive regulation of microtubule depolymerization / hyperosmotic salinity response ...stretch-activated, monoatomic cation-selective, calcium channel activity / blood vessel endothelial cell delamination / osmosensor activity / vasopressin secretion / positive regulation of striated muscle contraction / positive regulation of macrophage inflammatory protein 1 alpha production / calcium ion import into cytosol / negative regulation of brown fat cell differentiation / positive regulation of microtubule depolymerization / hyperosmotic salinity response / regulation of response to osmotic stress / positive regulation of chemokine (C-X-C motif) ligand 1 production / positive regulation of chemokine (C-C motif) ligand 5 production / cartilage development involved in endochondral bone morphogenesis / osmosensory signaling pathway / cellular hypotonic response / cortical microtubule organization / cellular hypotonic salinity response / multicellular organismal-level water homeostasis / positive regulation of vascular permeability / cellular response to osmotic stress / positive regulation of monocyte chemotactic protein-1 production / cell volume homeostasis / calcium ion import / cell-cell junction assembly / TRP channels / regulation of aerobic respiration / cortical actin cytoskeleton / positive regulation of macrophage chemotaxis / cytoplasmic microtubule / diet induced thermogenesis / beta-tubulin binding / microtubule polymerization / alpha-tubulin binding / response to mechanical stimulus / monoatomic cation channel activity / SH2 domain binding / filopodium / actin filament organization / adherens junction / protein kinase C binding / positive regulation of JNK cascade / calcium ion transmembrane transport / response to insulin / calcium channel activity / cilium / ruffle membrane / positive regulation of inflammatory response / positive regulation of interleukin-6 production / intracellular calcium ion homeostasis / calcium ion transport / actin filament binding / negative regulation of neuron projection development / lamellipodium / glucose homeostasis / actin binding / cellular response to heat / positive regulation of cytosolic calcium ion concentration / growth cone / actin cytoskeleton organization / microtubule binding / positive regulation of ERK1 and ERK2 cascade / calmodulin binding / response to hypoxia / apical plasma membrane / focal adhesion / lipid binding / protein kinase binding / negative regulation of transcription by RNA polymerase II / cell surface / endoplasmic reticulum / ATP binding / identical protein binding / membrane / metal ion binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.41 Å | |||||||||
 Authors Authors | Kwon DH / Lee S-Y / Zhang F | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: TRPV4-Rho GTPase complex structures reveal mechanisms of gating and disease. Authors: Do Hoon Kwon / Feng Zhang / Brett A McCray / Shasha Feng / Meha Kumar / Jeremy M Sullivan / Wonpil Im / Charlotte J Sumner / Seok-Yong Lee /  Abstract: Crosstalk between ion channels and small GTPases is critical during homeostasis and disease, but little is known about the structural underpinnings of these interactions. TRPV4 is a polymodal, ...Crosstalk between ion channels and small GTPases is critical during homeostasis and disease, but little is known about the structural underpinnings of these interactions. TRPV4 is a polymodal, calcium-permeable cation channel that has emerged as a potential therapeutic target in multiple conditions. Gain-of-function mutations also cause hereditary neuromuscular disease. Here, we present cryo-EM structures of human TRPV4 in complex with RhoA in the ligand-free, antagonist-bound closed, and agonist-bound open states. These structures reveal the mechanism of ligand-dependent TRPV4 gating. Channel activation is associated with rigid-body rotation of the intracellular ankyrin repeat domain, but state-dependent interaction with membrane-anchored RhoA constrains this movement. Notably, many residues at the TRPV4-RhoA interface are mutated in disease and perturbing this interface by introducing mutations into either TRPV4 or RhoA increases TRPV4 channel activity. Together, these results suggest that RhoA serves as an auxiliary subunit for TRPV4, regulating TRPV4-mediated calcium homeostasis and disruption of TRPV4-RhoA interactions can lead to TRPV4-related neuromuscular disease. These insights will help facilitate TRPV4 therapeutics development. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28978.map.gz emd_28978.map.gz | 59.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28978-v30.xml emd-28978-v30.xml emd-28978.xml emd-28978.xml | 15.5 KB 15.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_28978.png emd_28978.png | 116 KB | ||
| Filedesc metadata |  emd-28978.cif.gz emd-28978.cif.gz | 6.1 KB | ||
| Others |  emd_28978_half_map_1.map.gz emd_28978_half_map_1.map.gz emd_28978_half_map_2.map.gz emd_28978_half_map_2.map.gz | 59 MB 59 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28978 http://ftp.pdbj.org/pub/emdb/structures/EMD-28978 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28978 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28978 | HTTPS FTP |
-Validation report
| Summary document |  emd_28978_validation.pdf.gz emd_28978_validation.pdf.gz | 788.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_28978_full_validation.pdf.gz emd_28978_full_validation.pdf.gz | 787.9 KB | Display | |
| Data in XML |  emd_28978_validation.xml.gz emd_28978_validation.xml.gz | 12.3 KB | Display | |
| Data in CIF |  emd_28978_validation.cif.gz emd_28978_validation.cif.gz | 14.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28978 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28978 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28978 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28978 | HTTPS FTP |
-Related structure data
| Related structure data |  8fcaMC  8fc7C  8fc8C  8fc9C  8fcbC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_28978.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28978.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | main | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||
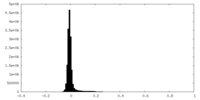
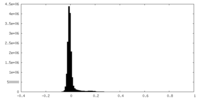
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: half A
| File | emd_28978_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half_A | ||||||||||||
| Projections & Slices |
| ||||||||||||
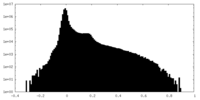
| Density Histograms |
-Half map: half B
| File | emd_28978_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half_B | ||||||||||||
| Projections & Slices |
| ||||||||||||
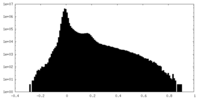
| Density Histograms |
- Sample components
Sample components
-Entire : The complex of human TRPV4 with RhoA
| Entire | Name: The complex of human TRPV4 with RhoA |
|---|---|
| Components |
|
-Supramolecule #1: The complex of human TRPV4 with RhoA
| Supramolecule | Name: The complex of human TRPV4 with RhoA / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 450 KDa |
-Macromolecule #1: Transient receptor potential cation channel subfamily V member 4
| Macromolecule | Name: Transient receptor potential cation channel subfamily V member 4 type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 102.057797 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MADSSEGPRA GPGEVAELPG DESGTPGGEA FPLSSLANLF EGEDGSLSPS PADASRPAGP GDGRPNLRMK FQGAFRKGVP NPIDLLEST LYESSVVPGP KKAPMDSLFD YGTYRHHSSD NKRWRKKIIE KQPQSPKAPA PQPPPILKVF NRPILFDIVS R GSTADLDG ...String: MADSSEGPRA GPGEVAELPG DESGTPGGEA FPLSSLANLF EGEDGSLSPS PADASRPAGP GDGRPNLRMK FQGAFRKGVP NPIDLLEST LYESSVVPGP KKAPMDSLFD YGTYRHHSSD NKRWRKKIIE KQPQSPKAPA PQPPPILKVF NRPILFDIVS R GSTADLDG LLPFLLTHKK RLTDEEFREP STGKTCLPKA LLNLSNGRND TIPVLLDIAE RTGNMREFIN SPFRDIYYRG QT ALHIAIE RRCKHYVELL VAQGADVHAQ ARGRFFQPKD EGGYFYFGEL PLSLAACTNQ PHIVNYLTEN PHKKADMRRQ DSR GNTVLH ALVAIADNTR ENTKFVTKMY DLLLLKCARL FPDSNLEAVL NNDGLSPLMM AAKTGKIGIF QHIIRREVTD EDTR HLSRK FKDWAYGPVY SSLYDLSSLD TCGEEASVLE ILVYNSKIEN RHEMLAVEPI NELLRDKWRK FGAVSFYINV VSYLC AMVI FTLTAYYQPL EGTPPYPYRT TVDYLRLAGE VITLFTGVLF FFTNIKDLFM KKCPGVNSLF IDGSFQLLYF IYSVLV IVS AALYLAGIEA YLAVMVFALV LGWMNALYFT RGLKLTGTYS IMIQKILFKD LFRFLLVYLL FMIGYASALV SLLNPCA NM KVCNEDQTNC TVPTYPSCRD SETFSTFLLD LFKLTIGMGD LEMLSSTKYP VVFIILLVTY IILTFVLLLN MLIALMGE T VGQVSKESKH IWKLQWATTI LDIERSFPVF LRKAFRSGEM VTVGKSSDGT PDRRWCFRVD EVNWSHWNQN LGIINEDPG KNETYQYYGF SHTVGRLRRD RWSSVVPRVV ELNKNSNPDE VVVPLDSMGN PRCDGHQQGY PRKWRTDDAP LENSLEVLFQ GPDYKDDDD KAHHHHHHHH HH UniProtKB: Transient receptor potential cation channel subfamily V member 4 |
-Macromolecule #2: (1aR,1bS,4aS,7aS,7bS,8R,9R,9aS)-9a-(decanoyloxy)-4a,7b-dihydroxy-...
| Macromolecule | Name: (1aR,1bS,4aS,7aS,7bS,8R,9R,9aS)-9a-(decanoyloxy)-4a,7b-dihydroxy-3-(hydroxymethyl)-1,1,6,8-tetramethyl-5-oxo-1a,1b,4,4a,5,7a,7b,8,9,9a-decahydro-1H-cyclopropa[3,4]benzo[1,2-e]azulen-9-yl decanoate type: ligand / ID: 2 / Number of copies: 4 / Formula: XS9 |
|---|---|
| Molecular weight | Theoretical: 672.931 Da |
| Chemical component information | 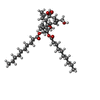 ChemComp-XS9: |
-Macromolecule #3: CHOLESTEROL HEMISUCCINATE
| Macromolecule | Name: CHOLESTEROL HEMISUCCINATE / type: ligand / ID: 3 / Number of copies: 4 / Formula: Y01 |
|---|---|
| Molecular weight | Theoretical: 486.726 Da |
| Chemical component information |  ChemComp-Y01: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | cell |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 281.15 K / Instrument: LEICA EM GP |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 81000 |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.41 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 244077 |
| Initial angle assignment | Type: OTHER |
| Final angle assignment | Type: OTHER |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)